Those who believe in clustered regularly interspaced short palindromic repeats (CRISPR) technology are cheering the recent approval for sickle cell disease by the United States Food and Drug Administration, all the more impressive given that the journey began in earnest only one decade ago. Still, drug developers have barely scratched the surface of CRISPR’s potential, and the expanding gene editing field still needs further regulations in terms of quality control.
Many pharmaceutical firms are turning their focus to the cost, scale, and access of CRISPR—and the technology to do so is already here. In the United States, San Diego, California-based CRISPR QC has developed a platform for real-time CRISPR analytics to help realize the full potential of gene editing. Its analytics platform is built on CRISPR-Chip technology that allows monitoring of affinity and kinetics, linking them to outcomes. Through better, faster understanding, CRISPR QC aims to drive down research and development (R&D) costs while enabling development of safe and accessible therapies.
The company was founded by CEO Ross Bundy and Kiana Aran, inventor of the foundational technology and an associate professor at University of California San Diego. In 2019, Aran and her team published data in Nature Biomedical Engineering on a field effect transistor for detecting genetic material. “We effectively built a CRISPR analyzer platform by anchoring a Cas enzyme to our sensor, and monitoring its performance in terms of binding to guide RNA and cleavage of target DNA, which can be invaluable in terms of CRISPR formulation evaluation, increasing efficiency and reducing cost,” said Aran, who heads CRISPR QC’s scientific advisory board (SAB). “RNA binding shapes CRISPR’s ability to edit genes. Understanding binding affinity and kinetics in real time lets us understand how changes to Cas proteins, guide RNAs, or their environment are linked to cell outcomes.”
Changing CRISPR dynamics
With the first CRISPR-based therapy reaching the market, the field is at a turning point. “Up until now, there was so much money flooding the industry, no one had to think much about cost or scale. Groups would screen hundreds of guide RNAs in in vivo studies, with no way of accurately predicting which ones worked best,” noted Aran. “But we know we can’t continue having each therapy cost $2–3 million per dose, and so efficiency has become key.”
The analytics platform enables researchers to move from qualitative to quantitative measurements, ensuring precision and reliability in gene editing outcomes (Fig. 1). This is a boon for therapeutics developers, synthetic biology companies, agricultural researchers and many others.
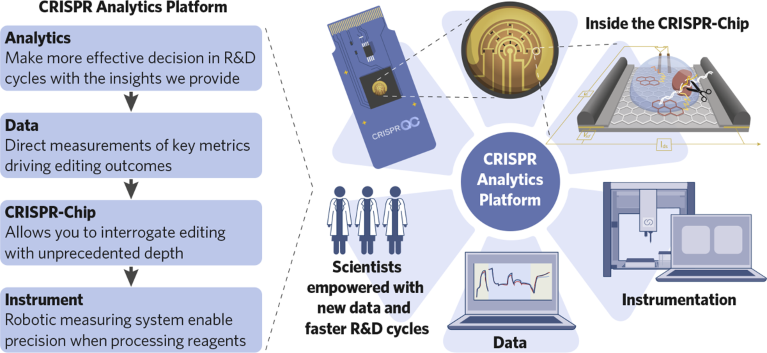
Fig. 1 | The complete workflow of the CRISPR Analytics Platform from sample input, through data interrogation, to actionable insights empowering scientists in advancing their research.
“The vast majority of CRISPR work today is based on using the Cas9 enzyme to do the same thing,” said Rodolphe Barrangou, a professor at North Carolina State University who has studied CRISPR applications for over a decade. “The unique part is selecting a guide RNA, and some companies sink huge resources and several years into screening them. But this platform accelerates screening efficiency at scale, with the added benefit of demystifying the guide RNA for regulators.”
The future may look different. “There are many groups designing new enzymes, or modifying existing ones for new applications,” said Aran. “Our platform has crucial applications for quality control there as well.”
CRISPR’s ease of use has attracted companies that lack deep genome editing expertise, which turn to the CRISPR QC analytics platform as a fast, cost-effective alternative to building robust internal capabilities. This includes pharmaceutical companies looking to in-license a single CRISPR-based asset, which can leverage the technology for a quality control tool throughout clinical development.
The platform has additional applications at every level of expertise, said Barrangou, who is on CRISPR QC’s SAB. “For the very advanced, scale is important, but R&D is challenging and resource-straining. Expanding to a new indication or target used to mean jumping back to the beginning of another long, laborious process.”
Partnerships and next steps
The company is committed to improving CRISPR efficiency, which includes a collaboration with the United States National Institute of Standards and Technology to establish quality control metrics for use across the industry. CRISPR QC is also eager to partner with groups looking to increase the efficiency of their CRISPR techniques and broaden the impact of gene editing applications.
Through its next funding round, the company is planning to enhance its data interrogation capabilities, building additional infrastructure to incorporate machine learning algorithms for analyzing additional parameters related to different cell lines and delivery methods.
“What’s remarkable is that ten years ago, the hype around CRISPR seemed impossible to meet, but in some ways we’ve already exceeded expectations,” said Barrangou. “Today’s milestones mark just the end of the beginning for CRISPR—and the first stage for CRISPR QC as well. This platform is going to help companies accelerate through the next decade as an entire ecosystem develops to keep the space growing.”


