Breast cancer is the most common cancer worldwide, with hormone receptor (HR)+/human epidermal growth factor receptor 2 (HER2)- being the most common subtype. Although targeted therapies offer the best hope for effective treatment, they show limited efficacy for early-stage HR+/HER2- patients due to a lack of novel predictive biomarkers. For this large and growing patient group, targeted therapies are also employed to combat advanced disease that is generally incurable—where they are merely extending lives with borrowed time. Fortunately, MeCo Diagnostics has identified a new approach for targeted therapy that appears to be highly effective in early-stage breast cancer, giving patients greater hope for lifelong remission after surgery. The venture-backed startup has developed a first-in-class, clinically validated predictive biomarker test to enable targeted antifibrotic therapy to prevent breast cancer metastasis.
A new treatment modality for breast cancer
One of the oldest observations in cancer biology is that tumors often grow stiffer than surrounding tissue, leading to a palpable lump—the most common indicator of disease. MeCo Diagnostics discovered that increased tumor stiffness, which is caused by fibrosis, can sometimes lead to a phenomenon called ‘mechanical conditioning’ in particular cancer cells, reprogramming them epigenetically to drive invasion and metastasis. However, the reason why only some tumors harbor these cells was not understood until 2021, when MeCo Diagnostics’ co-founders Adam Watson and Ghassan Mouneimne published a new understanding of the phenomenon1.
Fortuitously, the company also discovered that mechanical conditioning can be reversed with antifibrotic drugs, leading to the development of the Mechanical Conditioning (MeCo) Score, a first-in-class prognostic and predictive biomarker test to harness antifibrotic therapy. “The MeCo Score is an algorithm comprising 1,004 genes that are all mechanically regulated and metastasis associated. Our test identifies early-stage breast cancer patients who have a poor prognosis and may benefit from antifibrotic therapy,” explained Watson, MeCo Diagnostics’ CEO.
As a proof-of-concept, MeCo Diagnostics first showed that the antifibrotic drug Ofev (nintedanib) reduces breast cancer metastasis in animal models. Nintedanib seems particularly effective in HR+/HER2- tumors, representing ~70% of all breast tumors. In a recent prospective–retrospective phase 2 study, a high MeCo Score before treatment successfully predicted which patients benefitted from neoadjuvant antifibrotic therapy versus standard-of-care. Specifically, among patients with high MeCo Score HR+/HER2- tumors, the probability of relapse-free survival at 5 years after receiving neoadjuvant nintedanib plus chemotherapy was roughly double that of those receiving chemotherapy alone. Given that roughly half of all breast cancer patients have a high MeCo Score, ~35% of breast tumors may benefit from antifibrotic therapy, although this needs to be confirmed in a larger and fully prospective clinical trial. “These results show that MeCo Score testing can unlock a massive potential benefit for breast cancer patients,” said Watson. A prospective phase 2 randomized controlled trial is scheduled for 2024.
Huge new market opportunity for antifibrotic drugs
Since the MeCo Score is drug-agnostic, it can potentially leverage any investigational new antifibrotic drug as a viable therapy in early-stage breast cancer, thus facilitating label expansion into a huge new market—a strong rationale for pharma companies to reposition their antifibrotic drug candidates for breast cancer using the MeCo Score as a companion diagnostic (Fig. 1). “Investigational new drug (IND) antifibrotics developed for idiopathic pulmonary fibrosis (IPF), for example, have struggled in trials due to lack of predictive biomarkers,” said Watson. “But we can leverage these drugs for breast cancer patients, repositioning them for a much bigger market.”
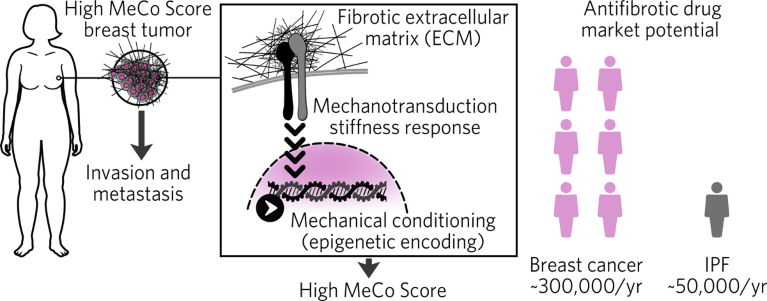
Fig. 1 | Lucrative market expansion potential. The MeCo Score is a drug-agnostic predictive biomarker for antifibrotic therapy that can be leveraged to unlock a much larger market than that of idiopathic pulmonary fibrosis (IPF).
Expanding the intended use of antifibrotic drugs to include breast cancer would represent an unusual circumstance in which a proposed new indication for a class of drugs is far more lucrative than the original indication; the US market for breast cancer is roughly six times that for IPF, with ~300,000 new cases of invasive breast cancer compared to only ~50,000 new cases of IPF diagnosed each year.
Accessing the vast breast cancer market with antifibrotics will only be possible with a predictive biomarker test such as the MeCo Score. MeCo Diagnostics is actively exploring multiple out-licensing opportunities for the MeCo Score with genomic-services providers and pharmaceutical companies: as a lab-developed test to harness nintedanib; as a companion diagnostic for a new antifibrotic drug; and/or as a prognostic test to monitor people with current or previously diagnosed disease for recurrence or response to therapy.
“The MeCo Score is the only clinically validated tool that can facilitate antifibrotic-drug repositioning for the large and growing breast cancer market. The goal is to reduce invasion and metastasis in patients before surgery, increasing their likelihood of lifelong remission,” said Watson. “Harnessing antifibrotics in early-stage disease to prevent breast cancer metastasis benefits all stakeholders: antifibrotic-drug developers, insurers, and patients alike.”


