Millions of people suffer from mild cognitive impairment (MCI)1, a major risk factor for dementia and Alzheimer’s disease that is commonly associated with anxiety2. Unfortunately, there are no disease-modifying treatments for MCI or dementia, and the selective serotonin-reuptake inhibitors (SSRIs) typically used to treat anxiety have limited effectiveness, inadequate response rates, and adverse side effects. This creates a pressing need for novel, more effective, and safer therapeutic interventions for these debilitating disorders. Enter Cognigenics, a biotechnology company specializing in advanced gene editing via intranasal delivery, focusing initially on treatments for MCI with chronic anxiety.
The platform
A substantial body of research points to hyperactivity in the amygdala and hippocampal regions of the brain in anxiety and memory-associated disorders, respectively. Harnessing genetic-neuroengineering techniques, Cognigenics uses short-hairpin RNA (shRNA) and clustered regularly interspaced short-palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) genome-editing tools to selectively target and modulate relevant neuronal receptors, enabling precise manipulation of the neural networks associated with anxiety and memory.
The genetic cargo is delivered intranasally to the brain via adeno-associated virus (AAV) vectors. The olfactory highway to the brain effectively bypasses the blood–brain barrier, while the use of AAV vectors ensures safe and efficient transportation of genetic material directly to neurons, facilitating gene-specific silencing in the limbic system.
Cognigenics’ initial therapeutics are designed to selectively downregulate the 5-hydroxytryptamine receptor 2A (5HT2A) post-synaptic neuronal receptors—a serotonin receptor subtype—in the brain via RNA or DNA genetic edits. The company’s current focus is on treating MCI and chronic anxiety, to meet the needs of a potentially huge market3. The underlying mechanism uses RNA interference (RNAi) to degrade 5HT2A messenger RNA (mRNA) and reduce 5HT2A receptor levels (Fig. 1). The observed behavioral effects are significantly reduced anxiety and enhanced memory4.
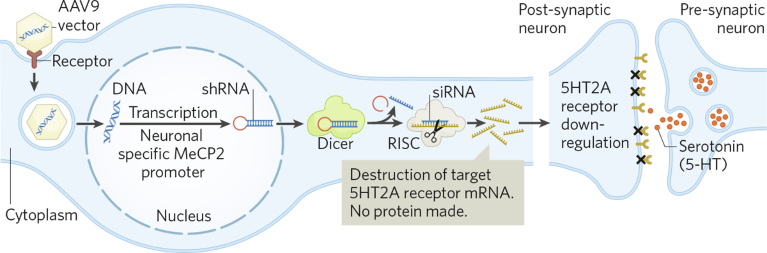
Fig. 1 | Targeted manipulation of neural networks. Adeno-associated virus 9 (AAV9) vectors enter cells via receptor-mediated uptake, shedding their coats to release DNA plasmids. The DNA, driven by a neuron-specific promoter, produces short-hairpin RNA (shRNA) targeting 5-hydroxytryptamine receptor 2A (5HT2A) messenger RNA (mRNA). The synthesized shRNA, processed into small-interfering RNA (siRNA), works with the RNA-induced silencing complex (RISC) to degrade 5HT2A mRNA, reducing receptor synthesis. This decrease in receptor presence on neuronal surfaces contributes to the observed anxiolytic and memory-enhancement effects. MeCP2, methyl-CpG-binding protein 2.
The company has confirmed these neuronal and behavioral outcomes via immunohistochemistry to trace where the genetic cargo diffuses in the brain; multi-electrode array electrophysiology to assess changes in neuronal performance; polymerase chain reaction (PCR) analyses to verify the genetic edits; and standard behavioral tests in mice and rats. In multiple experiments, the therapeutics resulted in up to 36% reduction in anxiety and 104% improvement in memory (as compared to controls)—surpassing the efficacy of standard first-line pharmaceutical interventions for anxiety and memory disorders, and without discernible side effects. In addition, experiments with appropriately revised genetic edits in human neurons in vitro show similar downregulation effects, indicating that the delivery technique and the expected neuronal regulation effects will be applicable to humans.
“These results suggest that our therapeutics could significantly reduce the burden of MCI and anxiety, paving the way for the potential reduction or eradication of these conditions at their neuronal source,” said co-founder and chairman Dean Radin, who anticipates that clinical trials will begin early in 2026.
SSRIs necessitate daily dosing, while most contemporary gene-based interventions for neurodegenerative conditions require either direct injection into the cerebrospinal fluid or intravenous administration, risking nerve damage and potential side effects from systemic distribution, explained Troy Rohn, director of preclinical studies. “In contrast, our approach uses intranasal delivery, which we now know can precisely target specific neuronal receptors and yield lasting therapeutic effects—an innovation in neuropsychiatric treatment that is also likely to improve patient compliance,” said Rohn. “With millions of people potentially benefitting, the projected cost per person is estimated to be comparable to existing SSRI drugs.”
Moreover, the versatility of this ground-breaking delivery platform is expected to enable customized therapeutic options aimed at addressing an extensive array of neurological conditions or behaviors, including chronic depression, dementia, and Alzheimer’s disease.
Partnering aspirations
Unlike most pharmaceutical companies, which are typically characterized by massive overhead costs and slow, risk-averse processes, Cognigenics operates with minimal overheads and is known for its rapid, entrepreneurial approach. The company achieves its efficiency by working closely with contract research and manufacturing organizations. This not only significantly slashes research and development (R&D) time and expenses, but also provides the company with a way to pursue multiple projects in parallel.
“We are addressing the complex challenges of MCI, anxiety, and, potentially, a broad spectrum of central nervous system (CNS) disorders at their root. Our intranasal genetic-medicine delivery platform provides a new class of therapeutics that is both patient-friendly and highly effective,” said Radin. “We stand at the forefront of a new era in mental-health treatments, offering hope and tangible solutions to those in need.”


