Alveolus Bio is pioneering inhaled delivery of live biotherapeutic products (LBPs) to reduce inflammation in patients with lung diseases. Built on science from a top US respiratory center, the biotech has created the first inhaled live biotherapeutic and advanced it to the cusp of clinical development as a treatment for both chronic and infectious lung disease.
The National Institutes of Health (NIH)-funded scientists and entrepreneurs Vivek Lal and Amit Gaggar, now Alveolus’ CEO and CMO, respectively, provided the scientific foundations for the biotech at their laboratories at the University of Alabama at Birmingham, one of the top funded US respiratory research centers in recent years. The researchers looked at the inflammation profiles of chronic lung disease patients and characterized their microbiomes via a multi-omics approach.
The work associated lower microbial diversity with susceptibility to chronic respiratory disease. Building on the finding, the collaborators isolated the specific bacteria linked to a healthier lung profile and identified the pathways they act on. These findings led to the creation of a live biotherapeutic candidate, AB1000, with the potential to treat chronic obstructive pulmonary disease (COPD), bronchopulmonary dysplasia (BPD), viral illnesses, and other lung diseases.
Delivering LBPs deep into the lung
For the active substance blend to effectively address lung neutrophilic inflammation, Alveolus needed to directly deliver it deep into the lungs. That posed two key challenges, namely, how to keep the bacteria alive during inhalation and how to enable deep delivery into the distal parts of the lung. Alveolus addressed both of these challenges through particle-engineering innovation.
Using a proprietary excipient-matrix formulation based on years of data on engineering particle characteristics for inhalation, the company achieved the particle size needed to get past the upper airways and travel deep into the lungs. The LBP formulation contains viable bacteria, achieving more than 80% viability conservation and a delivered dose of 86%. The tests showed a fine particle fraction of 74%, meaning most particles reach the deep lung.
The formulation has proven effective in preclinical tests. Alveolus created in vitro and in vivo models of chronic lung disease to test the candidate, which was also assessed in germ-free and humanized mouse models. The assessments suggest inhaled AB1000 reduces inflammation and supports the repair of lung tissue (Fig. 1).
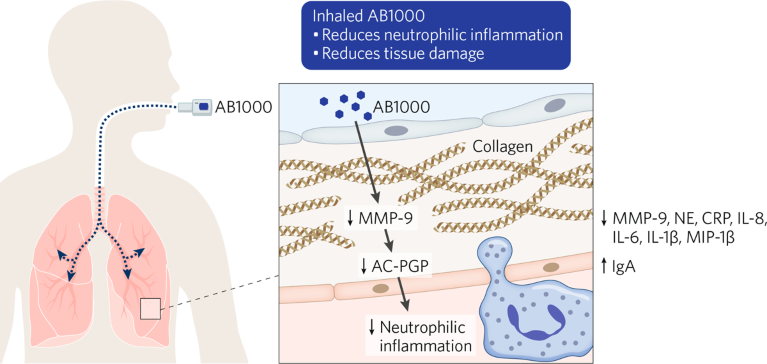
Fig. 1 | How AB1000 works. Particle-engineering innovation facilitates the delivery of AB100 directly into the lung to help reduce inflammation and restore lung tissue.
In COPD and BPD models, AB1000 reduced key pro-inflammatory markers such as matrix metallopeptidase 9 (MMP-9), C-reactive protein (CRP), and interleukin 1 beta (IL-1β), and increased anti-inflammatory markers. Comparative studies showed the LBP was as effective as steroids at reducing inflammation. Alveolus has also generated evidence of effects on fibrosis, linking the therapy to reductions in profibrotic markers, such as alpha smooth muscle actin (α-SMA) and type I collagen (COL1), as well as to increases in some antifibrotic markers.
The data suggest AB1000 is effective at reducing inflammation both during and after threat exposure and is able to regulate tissue repair and markers of fibrosis. Alveolus’ program is exploring that versatility by studying the candidate in COPD, BPD, pulmonary fibrosis, and injury secondary to viral illness. The company also has a small-molecule treatment for pulmonary hypertension in preclinical development.
Clinically validating a pioneering LBP
A phase 1b clinical trial of AB1000 is scheduled to start in 2023. The study will mark the start of a broader attempt to clinically validate the vast potential of Alveolus’ LBP and drug delivery platform technology.
Chronic lung disease is a major unmet medical need, with the US alone having 15 million COPD patients, 10 million neutrophilic asthma patients, and 15,000 annual BPD instances. Orphan lung diseases such as cystic fibrosis and idiopathic pulmonary fibrosis also cause severe symptoms in hundreds of thousands of patients.
The platform is equally applicable to infectious lung diseases, enabling Alveolus to use it to formulate drugs to protect patients against threats such as influenza, COVID-19, and pneumonia that can induce inflammation, tissue damage, and long-term fibrosis.Alveolus is now raising a Series A financing round to fund the phase 1b trial and the exploration of the potential of the platform. By building on its progress to date, the company will cement its position at the leading-edge of inhaled live biotherapeutic drug development and start addressing major unmet medical needs by decreasing inflammation in the lungs.


