AstraZeneca has a track record of doing things differently. The business development team has often broken with convention by pioneering novel deal structures and by challenging the consensus on hunting for best-in-class cancer medicines. The result is an industry-leading marketed oncology portfolio that is rapidly growing and redefining the standard of care across numerous cancer types, with half of current sales being derived from partnered medicines. It comes as no surprise, then, that AstraZeneca believes that partnering with innovators is key to continued leadership and the future success of its oncology business.
This focus on partnerships reflects a recognition that the best science cannot happen in isolation. Partnering allows AstraZeneca to build on its own expertise to access the best innovation, regardless of origin, to push the boundaries of science and rewrite the cancer treatment textbooks. Recognition of the vital importance of partnered programs keeps AstraZeneca agnostic to the source of innovation, ultimately for the benefit of cancer patients worldwide.
Much of what AstraZeneca has achieved for oncology patients in redefining standards of care can often be traced back to the deal table. AstraZeneca added a blood cancer medicine—the Bruton’s tyrosine kinase (BTK) inhibitor acalabrutinib—to its pipeline through the staged acquisition of Acerta Pharma in 2015. Trastuzumab deruxtecan, a HER2-targeted antibody–drug conjugate (ADC), joined AstraZeneca’s pipeline as part of a deal with Daiichi Sankyo in 2019 and shows promise against multiple HER2-expressing cancers. Equally, AstraZeneca has shown a willingness to partner its own portfolio programs, creating a leading franchise and turning a potential competitor into a collaborator by teaming up with Merck to maximize the value of its PARP inhibitor olaparib in multiple cancers.
What makes a good deal?
To improve the odds of value creation, filtering through the vast universe of potential collaboration opportunities is critical. The business development team at AstraZeneca uses a process of elimination, taking forward only those opportunities that score highly against scientific, cultural and financial criteria (Fig. 1). This process attempts to avoid the erosion of enterprise value through a mismatch in scientific vision, organizational values, and/or the decision-making process between prospective partners.
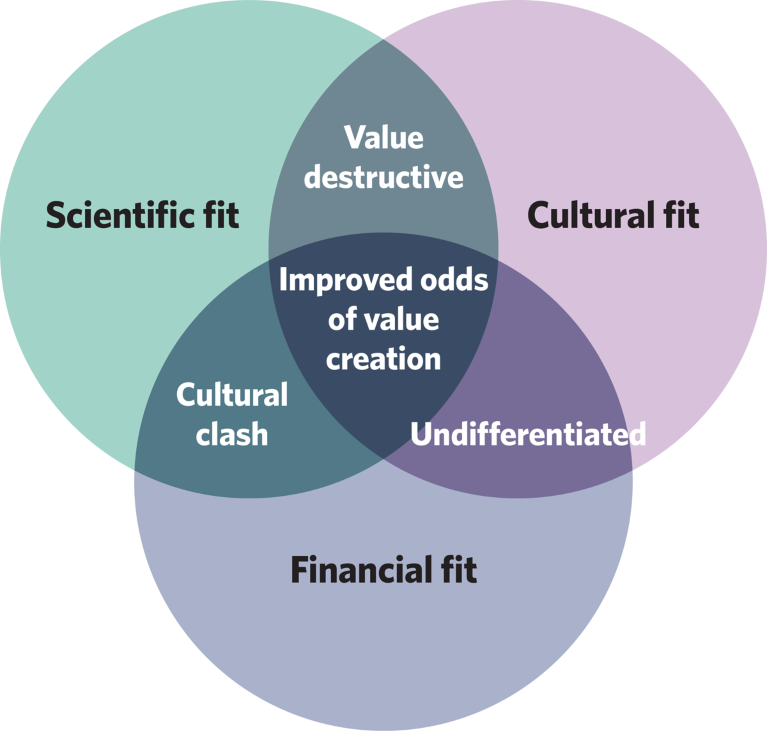
Fig. 1 | Factors influencing value creation within the AstraZeneca dealmaking model.
Put simply, good deals create value for all parties. However, that simple statement masks considerable complexity. Definitions of value differ between groups of stakeholders on both sides of the table, potentially resulting in misaligned incentives. Alignment is greatest when two similar organizations come together, for example when AstraZeneca and Merck functioned as peers in 2017 to develop and commercialize olaparib. In that case, the organizations on each side of the table are focused on innovating for patients, while combining unique portfolios and capabilities.
Today, misalignment of incentives in dealmaking between established pharma and innovative biotech companies can occur because there is unprecedented access to capital. Over the past decade, venture investment in oncology biotech has risen from approximately $800 million in 2011 to more than $40 billion in 2021. There is no doubt that the increase in investment will benefit patients. Because capital investment has increased much faster than the rate of company formation, companies are better funded than ever before. As a consequence, the reliance on pharma partners for non-dilutive capital and long-term partnership has, in some cases, been replaced by a focus on returns for investors through pharma acquisitions that occur relatively early in the biotech company’s life cycle. Today’s market conditions make it ever more important to ensure that potential biotech partners share a common understanding that creating new medicines for patients requires a long-term mindset.
AstraZeneca has a track record of partnering with like-minded, long-term-focused biotech companies at the vanguard of science. Recently announced collaborations include deals with Harbour BioMed, Scorpion Therapeutics, Fusion Pharmaceuticals and Accent Therapeutics. In each case, the biotech partner brought a highly specialized and focused set of capabilities that allow the respective companies to broaden their R&D efforts across new areas of oncology therapeutic discovery by partnering with a biopharmaceutical company with global reach. In turn, AstraZeneca offers innovative biotech companies the proven clinical development and marketing capabilities that are necessary to deliver medicines to patients.
How AstraZeneca pioneered a new trend in 50/50 oncology deals
AstraZeneca hasn’t been afraid to think differently and entertain collaboration structures that had long fallen out of favor to maximize the impact and reach of oncology medicines. Today, novel cancer medicines often require complex and capital-intensive clinical trials, across multiple cancer types, to provide the biggest benefit across the cancer community. Ultimately, three transformational collaborations exemplify the recognition that two companies can achieve far more together when working on medicines that provide a broad benefit to oncology patients.
The first deal saw AstraZeneca team up with Merck to co-develop and co-commercialize its PARP inhibitor olaparib across multiple cancer types. AstraZeneca received $1.6 billion upfront as part of an agreement that saw Merck agree to share the cost of developing olaparib as a monotherapy and each party funding development of its own checkpoint inhibitor combination therapies with durvalumab and pembrolizumab, respectively.
With olaparib in development in 14 indications at the time of the deal, AstraZeneca saw that the addition of Merck’s resources and expertise to its own capabilities was the best way to realize the medicine’s vast potential. The bet paid off. Revenues for olaparib have grown from $297 million in 2017, the year of the Merck deal, to nearly $3 billion in 2021—a period defined by a series of approvals in four different cancer types. The deal has since served as a blueprint for agreements between other companies, and the medicine is the leading PARP inhibitor worldwide by sales.
AstraZeneca next applied the 50/50 model to a pair of in-licensing deals with Daiichi Sankyo. The deals gave AstraZeneca stakes in ADCs directed at HER2 and TROP2. ADCs have come of age since the first Daiichi Sankyo deal in 2019, leading to an increase in interest from big pharma, but AstraZeneca’s $1.35 billion upfront payment for rights to the candidate DS-8201, also known as trastuzumab deruxtecan (T-DXd), was a bold bet at the time. To fund the deal, AstraZeneca issued a $3.5 billion equity placement, a highly unusual mechanism to fund a pharma licensing transaction. The move paid off, as today T-DXd showcases what next-generation ADCs are potentially capable of delivering for patients.
In 2020, AstraZeneca became the first company to enter into a global deal for a TROP2-targeted ADC when it agreed a second deal with Daiichi Sankyo, for DS-1062, also known as datopotamab deruxtecan (Dato-DXd). Moving early and decisively on the partnering process for Dato-DXd enabled AstraZeneca to secure rights to a potential new treatment for multiple cancer types, while again providing the resources of two large organizations to provide a comprehensive development plan that neither company alone would be capable of executing.
In an industry in which following trends is the safe option for business development teams, AstraZeneca has repeatedly struck out on its own, spotting opportunities before they are obvious and taking smart risks to enter untapped territories ahead of its peers.
The Merck and Daiichi Sankyo agreements demonstrate AstraZeneca’s willingness to look for value in unconventional places while making antiquated deal structures once again fashionable. AstraZeneca’s operating principle is this: how do we ensure that each collaboration delivers more than just the sum of each company’s parts, especially given the complexity and costs associated with every development program? With frothy biotech valuations limiting the financial attractiveness of actionable opportunities, AstraZeneca’s ability to find ways to enhance the value of potential medicines in unconventional places has proven invaluable in recent years and a rich ground for industry-leading business development scouting activities.

AstraZeneca is a disciplined dealmaker
The current success enjoyed by AstraZeneca’s oncology R&D portfolio creates a naturally high bar for external partnering opportunities. In addition, the revenue growth profile enjoyed by the company today relieves the all-too-common tendency to become a forced buyer in a competitive market. Furthermore, a disciplined approach by the business development team quickly removes opportunities that don’t score highly against scientific, cultural and financial criteria. What remains, then, are opportunities that have increased odds of enhancing enterprise value.
What’s next? Dealmaking in a changing landscape
Urgency, conviction and an organizational structure that maximizes agility are the key factors that have enabled the successful dealmaking track record at AstraZeneca. Creativity and the willingness to think differently and innovatively set AstraZeneca apart from its peers in competitive situations, such as the two ADC collaborations with Daiichi Sankyo.
AstraZeneca’s oncology business development team will need to keep moving quickly and creatively to thrive in the years to come. As a therapeutic area, oncology is among the most competitive, and requires organizational scale, which can only be achieved through the marriage of a productive internal R&D operation and the continuous sourcing of external innovation. In other words: the best science does not happen in isolation.
Veeva ID: Z4-43372. Date of preparation: April 2022


