
Credit: Nina Bondarchuk / Alamy Stock Photo
As lockdowns aimed at handling the COVID-19 pandemic swept the world during the first quarter of 2020, new dealmaking trends began to emerge. Competitors partnered for the first time, never-before-seen deals were penned and other deals were agreed in record time. With the help of biotech database DealForma, we overview dealmaking in a year dominated by the pandemic, including a highlight of activity in the respiratory disease area.
The deals landscape—aside from those fast-tracked to tackle COVID-19—almost ground to a halt as global lockdowns struck in March and April. The number of mergers and acquisitions (M&As) also declined as the pandemic took hold, but recovered and started to increase in frequency as some restrictions lifted. Venture investment continued to flow throughout 2020, and some therapy areas, such as immuno-oncology (IO), continued to thrive despite the pandemic; the number of IO drugs in development increased by 22% compared with 2019 (Nat. Rev. Drug Discov. 19, 751–752; 2020).
High-value licensing deals
As some lockdowns began easing from May, dealmaking started to accelerate, with larger upfront payments being made for earlier-stage development deals. In July, the highest value deal of 2020 was signed between AstraZeneca and Daiichi Sankyo for the development and marketing of DS-1062, an antibody–drug conjugate (ADC) targeting TROP2 that was then in phase 1 development to treat multiple tumor types including lung and breast cancer (Table 1). AstraZeneca made an upfront payment of $1 billion to Daiichi Sankyo and committed to paying up to $5 billion in commercial and sales milestones.

Table 1 | Top ten R&D biopharma therapeutics and platforms partnerships in 2020 by deal value.
Table 1 shows that many of the year’s highest-value deals were focused on oncology treatments. The second largest deal was a partnership between Merck & Co. and Seattle Genetics Inc. (now known as Seagen) in a development deal for ladiratuzumab vedotin, a LIV1-targeting ADC in phase 2 trials to treat solid tumors including breast cancer. The deal involved Merck buying a $1 billion equity stake in Seagen, with further payment milestones down the line worth up to $2.6 billion. Bispecific antibodies—another platform that has attracted a lot of recent attention—were the focus of the third most valuable deal, in which AbbVie agreed to pay Genmab $750 million upfront and up to $3.15 billion in milestones to partner on bispecific antibodies, including the leading candidate epcoritamab, which is in phase 1/2 development for blood cancers.
Outside of oncology, there were a couple of high-value deals focused on neurodegenerative disorders, both involving Biogen. In February, the company announced a deal worth up to $2.7 billion to gain rights to license, develop and commercialize preclinical gene regulation therapies identified by Sangamo Therapeutics for neurological diseases including Alzheimer and Parkinson diseases. And in August, Biogen teamed up with Denali Therapeutics in a deal worth $2.1 billion to co-develop small-molecule drugs for Parkinson disease that target LRRK2 gene mutations, including a phase 1 small-molecule LRRK2 inhibitor DNL151.
Slow-start M&As accelerate
M&A activity started steadily in 2020 but at lower volumes than in Q1 2019. Mega mergers were also scarce, but, as the year progressed, activity started to pick up. The largest acquisition of 2020 was Gilead Science’s purchase of Immunomedics for $21 billion in September, which gave Gilead access to the TROP2-targeted ADC Trodelvy (sacituzumab govitecan), a rival to Daiichi’s DS-1062 that was approved by the US Food and Drug Administration (FDA) in April to treat triple-negative breast cancer (Table 2).
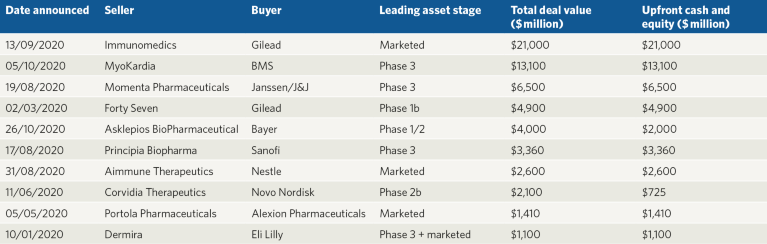
Table 2 | Top ten biopharma merger and acquisitions (M&As) in 2020.
In October, Bristol Myers Squibb (BMS) purchased cardiovascular therapeutics company MyoKardia for $13 billion, giving BMS MyoKardia’s lead drug candidate mavacamten. The myosin inhibitor is in late-stage development for hypertrophic cardiomyopathy and is expected to be submitted for FDA approval in 2021. Late-stage assets also drove a number of other M&As in the immunology area. Janssen Pharmaceuticals signed the third highest value acquisition when it bought Momenta Pharmaceuticals, gaining the autoimmune disease candidate nipocalimab, an anti-FcRn monoclonal antibody that has completed phase 2 trials for myasthenia gravis, which is also predicted to have blockbuster potential. Sanofi’s $3.7-billion acquisition of Principia Biopharma brings a portfolio of candidates for autoimmune disease, including the BTK inhibitor rilzabrutinib currently in phase 3 trials. Finally Palforzia, the first approved oral immunotherapy for peanut allergy, no doubt fuelled Nestle’s $2.6 billion acquisition of Aimmune Therapeutics in August.
A number of significant medical device industry M&As were also signed this year including Teladoc’s $18.5-billion merger with Livongo Health in the virtual care field, which has become increasingly important given the challenges that COVID-19 has posed for in-person care. Teladoc, a specialist in telehealth, announced the merger in August and it was completed by late October. Three of the other top five M&As focused on cancer diagnostics, with the largest being Siemens Healthineers’ $16.4 billion all-cash acquisition of oncology systems and software company Varian Medical Systems.
COVID-19 takes hold
Many partnerships were established in 2020 to tackle the coronavirus pandemic, including vaccine development, diagnostics and treatments to test for and manage COVID-19 (Biopharma Dealmakers B18–19; June, 2020).
Deals started to trickle in through Q1, beginning as early as January when the Coalition for Epidemic Preparedness and Innovations—set up in 2017 to coordinate the work of public, private, philanthropic and civil organizations for vaccine development to stop epidemics—partnered with both Moderna Therapeutics and Curevac among others to provide funding to develop their respective vaccine candidates. The US Biomedical Advanced Research and Development Authority also funded a host of companies developing a vaccine candidate.
The volume of COVID-19 deals gained momentum by the end of Q1. Sanofi and GlaxoSmithKline joined forces for the first time in a partnership that combined Sanofi’s spike protein antigen with GlaxoSmithKline’s adjuvant technology. A number of the partnerships on vaccine candidates that are now close to the end of phase 3 trials were also signed in the first half of the year, including one signed in April between AstraZeneca and the University of Oxford on their adenovirus vector-based vaccine and another between Pfizer and BioNTech signed in March on their mRNA-based vaccine that has recently become a front runner in the vaccine race after announcing promising phase 3 trial results.
Partnerships on antibody candidates have also been prominent (Biopharma Dealmakers, B3–5; September 2020), such as those Regeneron signed with the US government public–private partnership Operation Warp Speed to supply its antibody candidate REGN-COV2 and with the National Institute of Allergy and Infectious Diseases (NIAID) to conduct COVID-19 prevention trials. Partnerships have been instrumental in tackling COVID-19 and have accelerated the development of treatments and vaccines.
Respiratory disease deals in 2020
The COVID-19 pandemic caused some areas to see a decline in deals, but the number of deals in the respiratory disease area increased substantially, as COVID-19 infection can cause severe respiratory disease and exacerbate existing conditions such as asthma and chronic obstructive pulmonary disease (COPD). According to DealForma, more than 300 respiratory disease-related deals—many focused on COVID-19—have been signed since the start of 2020, compared to 44 deals announced in 2019.The majority of respiratory disease deals were signed at the platform/discovery stage (Fig. 1) as companies looked to develop vaccine candidates and antibody treatments based on emerging knowledge about the virus. Most deals were signed for vaccines, followed by small molecules, antibodies and diagnostics (Fig. 2).
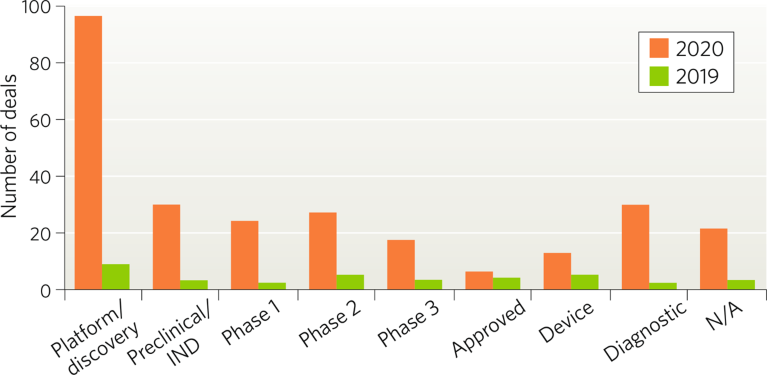
Fig. 1 | Respiratory disease type deals by development stage (January 2019–October 2020). IND, investigational new drug.
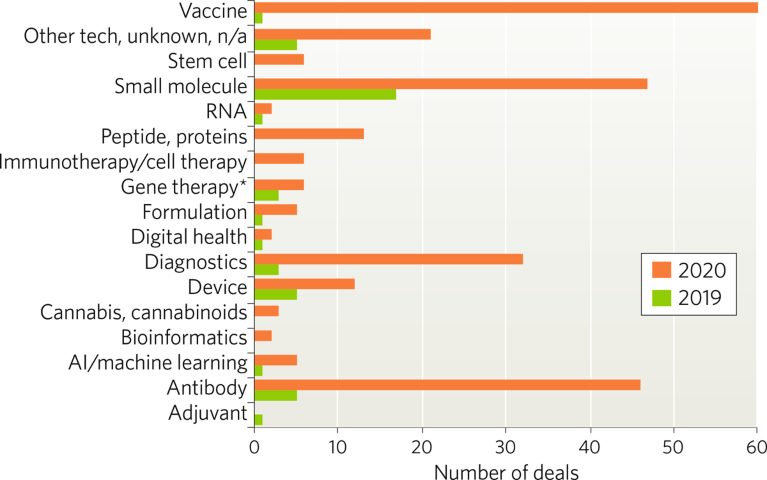
Fig. 2 | Respiratory disease deals by primary technology type (January 2019–October 2020). *Deals also include those covering genomics and gene-editing. AI, artificial intelligence.
Looking past COVID-19, many companies have signed other respiratory disease-related deals (Table 3), although some are not focused on respiratory diseases alone. Oneness Biotech granted LEO Pharma worldwide rights to develop and commercialize FB825, a monoclonal antibody that targets membrane-bound IgE, in a deal potentially worth up to $610 million. LEO Pharma will take over development responsibilities for FB825, once a phase 2a trial run by Oneness in atopic dermatitis and a phase 2a trial in allergic asthma run by Microbio Shanghai are completed. And in a deal worth approximately $268 million, Curadev granted Bayer rights to develop small-molecule STING antagonists for the treatment of lung, cardiovascular and other inflammatory diseases.
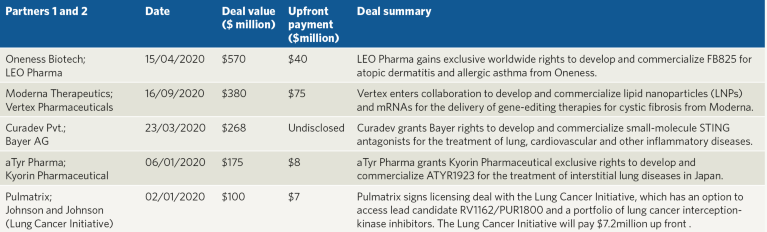
Table 3 | Selected respiratory deals by value in 2020.
Deals involving technologies to deliver novel therapies to the lung have also been signed. Vertex Pharmaceuticals partnered with Moderna Therapeutics to develop and commercialize lipid nanoparticles and mRNAs for the delivery of gene-editing therapies for the treatment of cystic fibrosis in deal worth up to $455 million. Within the deal, Moderna will develop a lipid nanoparticle delivery system and mRNAs, and Vertex will manage the preclinical, clinical development and commercialization of the therapies.
Finally, via a deal signed in March with undisclosed terms, Vir Biotechnology has collaborated with Alnylam Pharmaceuticals to explore the development of ‘inhaled RNA’, focusing on lung-targeted small interfering RNA conjugates against SARS-CoV-2 and other coronaviruses (Nat. Biotech. 38, 1110–1112; 2020).

