Resistance to conventional and targeted therapy is a major cause of failure of anti-cancer treatment. Although immune-checkpoint inhibitors (CPIs) mobilize the immune system to combat cancer cells, they have demonstrated sustainable therapeutic results in only a relatively small proportion of cancer patients. Not surprisingly, the fight against cancer has now tilted fully toward the evaluation of combinations of drugs in select patient populations. Furthermore, improved understanding of the molecular and epigenetic drivers of immune evasion and resistance is underpinning efforts to develop therapies that can selectively kill drug-tolerant, immune-evasive cancer cells and prevent disease recurrence.
During embryonic development in vertebrates, epithelial cells convert to mesenchymal fibroblast-like cells through a process called epithelial– mesenchymal transition (EMT), thereby giving rise to different structures and organs. While EMT is seldom activated in healthy adults, there is evidence that it has an important role in tumor progression, giving cancer cells the ability to disarm the body’s anti-tumor defenses, evade the immune system, become resistant to anti-cancer drugs, escape from the primary tumor and spread through the body. Not surprisingly, EMT has become the focus of substantial interest among cancer researchers.
Research has shown that the Axl receptor, which is expressed on the surface of cells, is a key mediator of EMT in solid cancers. Moreover, a recent paper in Nature 1 described how some cells are able to evade the immune system and acquire resistance to cancer drugs via an Axl mediated mechanism resulting from an epigenetic upregulation of the Axl receptor in response to the cells’ hostile microenvironment. These so-called Axl jackpot cells validate BerGenBio’s view that Axl signaling is a fundamental mechanism of cancer cell survival and escape.
“We have seen that Axl expression correlates with the worst overall survival of cancer patients. If you study the survival plots of most cancers—breast, lung, AML, pancreatic, etc—the patient population with the worst survival outlook are those with a strong Axl expression in their tumors,” explained BerGenBio CEO Richard Godfrey.
Axl is a member of the receptor tyrosine kinase (RTK) class, which is already a source of many essential cancer targets, with several important drugs, such as Roche’s EGFR-targeting Tarceva (erlotinib), acting through RTK modulation.
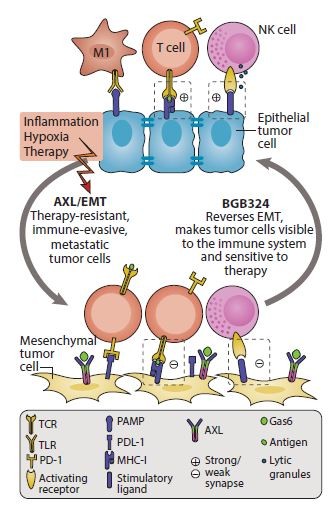
Figure 1: BGB324 in action. BGB324 increases anti-tumor immunity through reversal of EMT and increase of anti-tumor immune response
BerGenBio is focusing on the inhibition of Axl signaling. “By inhibiting Axl signaling we expect that cells that have undergone EMT will regain their epithelial characteristics, making them more visible to the immune system, regain sensitivity to conventional and targeted therapies and be less metastatic,” added Godfrey.
Significantly, the company has elucidated a compelling multifaceted mechanism of action (MoA) of Axl-mediated immune evasion by cancer cells, and demonstrated a strong preclinical rationale for combining the compound BGB324 with CPIs. It has shown that this drug combination increases the activation and infiltration of cytotoxic T lymphocytes (CTLs) and natural killer cells and significantly improves treatment outcomes. The MoA centers on the tendency of mesenchymal cancer cells to express significantly more immune-checkpoint ligands, which activate the immune checkpoints on the CTLs and therefore mediate immune evasion by the cancer cells. When Axl is blocked by BGB324, the mesenchymal phenotype is inhibited (and reversed), which reduces the checkpoint-ligand expression by the cancer cell, thus preventing checkpoint activation and allowing for greater immune-mediated cancer cell death (Fig. 1). Compelling preclinical data support the rationale for the combination of BGB324 with CPI in multiple cancer settings, including aggressive mammary models and adenocarcinoma non-small-cell lung cancer (NSCLC).
In early 2017 the company established two clinical collaborations with Merck & Co. (known as MSD outside the United States and Canada), whereby BerGenBio sponsors two separate phase 2 clinical trials of BGB324 with Merck & Co.’s blockbuster CPI Keytruda (pembrolizumab), “These collaboration agreements confirm that Merck & Co., a leader in checkpoint inhibitors, is eager to explore combination therapies with innovative new drugs with strong scientific rationale,” added Godfrey.
These agreements underpin two phase 2 multicenter studies of a BGB324–Keytruda combination in up to 48 patients with previously treated advanced adenocarcinoma of the lung and up to 56 patients with previously treated metastatic triple-negative breast cancer. Furthermore, the company is supporting a real-world combination study in melanoma patients. Other ongoing phase 2 studies include single and combination studies in acute myeloid leukemia (AML)/myelodysplastic syndrome (MDS), combination with Tarceva in EGFR-driven NSCLC patients, and combination with Taxotere (docetaxel) in other NSCLC patients.
The company has reported safety data from a phase 1 healthy-volunteer study, as well as early efficacy signals from its phase 1b single-agent study in AML/MDS and phase 1b combination study with Tarceva in NSCLC. Both partial and complete remissions were reported in AML/MDS patients, as well as an overall clinical benefit rate of 32% and excellent responder correlation with the company’s biomarkers. In stage 4 metastatic NSCLC patients, treatment with BGB324 as a single agent led to one-year progression-free survival in 25% of patients. And when given BGB324 in combination with Tarceva, 50% of patients saw meaningful clinical benefit for more than four months, and one patient’s partial remission is ongoing for nearly two years.
The ongoing phase 2 studies will report out in 2018, along with parallel companion diagnostic studies that are intended to facilitate an enriched phase 3 program and a precision medicine approach to reimbursement.
BerGenBio intends, either alone or in collaboration with a partner, to develop and commercialize BGB324 through to marketing approval for a variety of cancers.


