Vaccines have a tremendous impact on public health, and their importance has been emphasized by the COVID-19 pandemic. Here, we provide an overview of the current state of research and development (R&D) on prophylactic vaccine candidates for infectious diseases globally.
Technology platforms
As of 1 January 2023, the global vaccine R&D landscape includes 966 candidates, among which 23% (220) are traditional inactivated or attenuated vaccines (Fig. 1a,b). Advances in molecular technologies have led to the development of other platforms, such as recombinant protein vaccines, nucleic acid vaccines and viral vector vaccines, which have further diversified the pipeline.
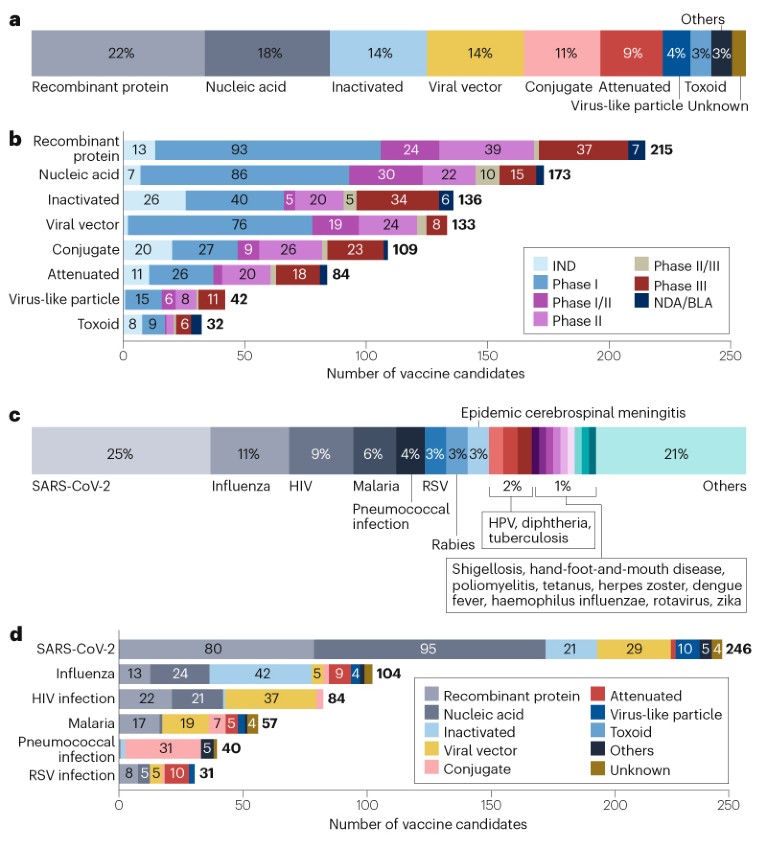
Fig. 1 | Landscape of vaccine candidates by technology platform, R&D phase and disease. a, The 966 vaccine candidates were classified into the categories shown based on the underlying technology platforms. Products with inadequate information for classification were included in an ‘Unknown’ group, and those not fitting into the main groups were included in an ‘Others’ group. b, All candidates excluding ‘Unknown’ and ‘Others’ are shown in the bar chart, classified by R&D phase. c, Proportion of vaccine candidates against different diseases. Diseases with less than ten candidates were included in the ‘Others’ group. d, Candidates for the top six diseases for vaccine development, by technology platform. HPV, human papilloma virus; IND, investigational new drug application; RSV, respiratory syncytial virus. See Supplementary information for details.
Recombinant protein vaccines account for the largest proportion of the pipeline at 22% (215 candidates; Fig. 1a,b), supported by their well-understood safety profile, stability and ease of manufacturing. Almost 100 recombinant vaccine candidates are in phase I development, the highest number at this stage among all the platforms.
The successful launch of SARS-CoV-2 mRNA vaccines has built momentum for nucleic acid vaccine platforms, which include RNA and DNA vaccines. Such platforms now make up the second largest segment of the overall pipeline, at 18% (173 candidates; Fig. 1a,b). Owing to the flexibility of these platforms in developing vaccine candidates for pathogens with high variability in the target antigens, many of these candidates are being developed for such pathogens, including SARS-CoV-2 (95 candidates), influenza (24 candidates) and HIV (21 candidates).
Viral vector vaccines (133 candidates; 14%; Fig. 1a,b) have also garnered attention in recent years, owing to their potential to induce robust and durable immune responses. Various types of viral vectors are being used, including adenoviruses, retroviruses, lentiviruses and poxviruses. In particular, adenoviral vectors (82 candidates) have been widely applied in the development of vaccines for diseases including Ebola, HIV, influenza and SARS-CoV-2. To circumvent the limitation of pre-existing immunity to adenovirus type 5 (Ad5), versatile adenoviral serotypes have been developed, such as Ad26, Ad35 and Ad11.
Conjugate vaccines, which are the next largest group (109 candidates; 11%; Fig. 1a,b), have often been developed against pathogens such as meningococcus, pneumococcus and haemophilus influenzae. These vaccines are based on covalent linkage of immunogenic protein carriers (mostly tetanus toxoid, diphtheria toxoid or group B meningococcal outer membrane protein) to capsular polysaccharides or peptides to enhance immunogenicity and stability.
Diseases
The top three diseases for vaccine development are all caused by viruses: SARS-CoV-2 (246 candidates; 25%), influenza (104 candidates; 11%) and HIV (84; 9%) (Fig. 1c,d; Supplementary Fig. 1).
SARS-CoV-2. In addition to over 50 vaccines that have received market approval or emergency use authorization (not included in this analysis), another 64 candidates had entered phase III or had a regulatory application submitted, of which 47% were mRNA vaccines. In the current pipeline, at least 14 nasal vaccines are in development, which are expected to stimulate immunity in the respiratory mucosa and reduce transmission.
HIV infection. The high variability of the viral genome and the high level of glycosylation of the HIV envelope glycoprotein (gp), which often induces immune evasion, have hindered the development of successful HIV vaccines. However, there is hope of stimulating the production of broadly neutralizing antibodies (bnAbs) by targeting conserved regions of envelope proteins with little variation between HIV strains, such as gp160, gp41 and gp120. Novel platforms such as viral vectors and mRNA offer a promising avenue for HIV vaccine development. For example, two mRNA vaccines to induce the production of bnAbs are now in a phase I trial (NCT05001373).
Influenza. In contrast to the dominance of novel technologies in HIV vaccine development, 40% of influenza vaccine candidates were inactivated vaccines (Fig. 1d). Given the variety of influenza subtypes from antigenic drift, universal vaccines are being increasingly developed to reduce the need for frequent vaccination. The vaccines were designed based on highly conserved epitopes in the viral haemagglutinin, neuraminidase or other proteins. At the time of analysis, six universal influenza vaccine candidates were in phase III trials.
Other diseases. Beyond the three diseases above, there are a substantial number of vaccines being developed for respiratory syncytial virus (31 candidates; 3%), supported by recent breakthroughs in targeting stable pre-F proteins. Notably, two recombinant protein vaccines, PF-06928316 and GSK3844766A, resulted in >80% protection in phase III trials, and received FDA approval in 2023, after the cut-off date for the analysis. An mRNA vaccine (mRNA-1345) has received breakthrough therapy designation from the FDA after also showing >80% protection in a phase III trial.
Non-viral pathogens such as malaria (57 candidates; 6%) and pneumococci (40 candidates; 4%) also represent a significant area of interest (Fig. 1c,d). Conjugate vaccines are the primary focus for pneumococcal bacteria, while recombinant proteins and viral vectors are the main platforms for malaria vaccines. Vaccines are also being developed to prepare for potential future outbreaks of diseases such as Ebola.
R&D distribution
Vaccine R&D is mainly concentrated in the USA (355 candidates), China (271 candidates) and western Europe (144 candidates) (Fig. 2a), in part due to their strong R&D capabilities and regulatory policy support. Some differences in technology platform preferences have been observed in these regions; the US pipeline features more nucleic acid vaccines, whereas the pipeline in China has more inactivated vaccines and fewer viral vector vaccines than the USA and western Europe. The majority (68%) of the candidates are being developed independently or collaboratively by private companies/industry, while 25% are being developed by academic or other non-profit organizations (Fig. 2b). Notably, candidates against HIV and malaria are being developed mostly by academic or other non-profit organizations.
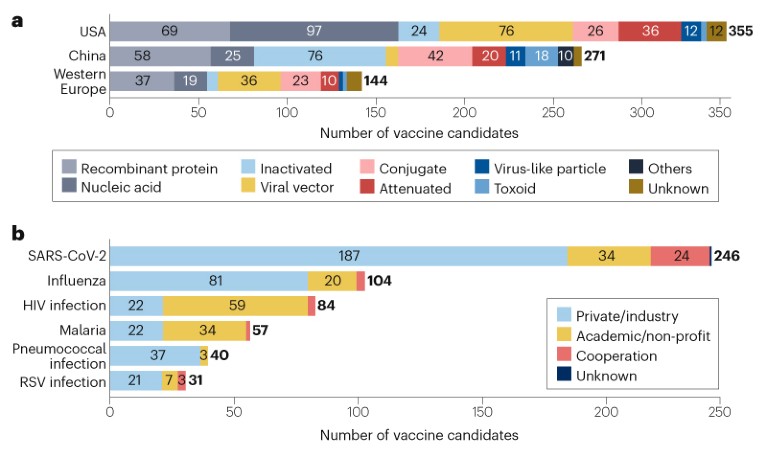
Fig. 2 | Distribution of vaccine candidates by geographic location and type of developer. a, Vaccine candidates with developers from the USA, China and western Europe, categorized by technical platform. b, Candidates for the top six diseases for vaccine development, by type of developer. See Supplementary information for details.
Outlook
The success of vaccine development relies heavily on the identification of effective antigens and the use of appropriate technology platforms. In addition, international collaboration and coordinated efforts are crucial to efficiently achieve these goals. The COVID-19 pandemic has highlighted the importance of global cooperation in addressing public health emergencies, and has demonstrated the potential benefits of sharing resources and expertise to accelerate vaccine development and deployment. This includes sharing scientific resources and expertise, collaborating on R&D, and establishing coordinated mechanisms for outbreak preparedness and response.
