Abstract
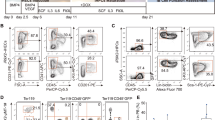
Effector B cells are central contributors to the development of autoimmune disease by activating autoreactive T cells, producing pro-inflammatory cytokines and organizing ectopic lymphoid tissue. Conversely, IL-10-producing regulatory B (Breg) cells have pivotal roles in maintaining immunological tolerance and restraining excessive inflammation in autoinflammatory disease. Thus, regulating the equilibrium between antibody-producing effector B cells and Breg cells is critical for the treatment of autoimmune disease. In this study, we investigated the effect of human palatine tonsil-derived mesenchymal stem cells (T-MSCs) on estradiol (E2)-induced B-cell responses in vivo and in vitro. Transplantation of T-MSC into E2-treated mice alleviated B-cell-mediated immune responses and increased the population of IL-10-producing Breg cells. T-MSCs regulated the B-cell populations by producing Epstein–Barr virus (EBV)-induced 3 (EBI3), one of the two subunits of IL-35 that is the well-known inducer of Breg cells. We demonstrate a critical role of EBI3 (IL-35) in vitro by depleting EBI3 in T-MSCs and by adding exogenous IL-35 to the culture system. Taken together, our data suggest that IL-35-secreting MSCs may become an attractive therapeutic to treat B-cell-mediated autoimmune diseases via expanding Breg cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Gatto D, Brink R . The germinal center reaction. J Allergy Clin Immunol 2010; 126: 898–907.
Shlomchik MJ, Weisel F . Germinal center selection and the development of memory B and plasma cells. Immunol Rev 2012; 247: 52–63.
Francisco LM, Sage PT, Sharpe AH . The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010; 236: 219–242.
Luckey D, Medina K, Taneja V . B cells as effectors and regulators of sex-biased arthritis. Autoimmunity 2012; 45: 364–376.
Vancsa A, Szabó Z, Szamosi S, Bodnár N, Végh E, Gergely L et al. Longterm effects of rituximab on B cell counts and autoantibody production in rheumatoid arthritis: use of high-sensitivity flow cytometry for more sensitive assessment of B cell depletion. J Rheumatol 2013; 40: 565–571.
Woods M, Zou YR, Davidson A . Defects in germinal center selection in SLE. Front Immunol 2015; 6: 425.
Mauri C, Blair PA . Regulatory B cells in autoimmunity: developments and controversies. Nat Rev Rheumatol 2010; 6: 636–643.
Mauri C, Bosma A . Immune regulatory function of B cells. Annu Rev Immunol 2012; 30: 221–241.
Borkowski AW, Kuo IH, Bernard JJ, Yoshida T, Williams MR, Hung NJ et al. Toll-like receptor 3 activation is required for normal skin barrier repair following UV damage. J Invest Dermatol 2015; 135: 569–578.
Miyagaki T . Regulatory B cells in human autoimmune diseases. Nihon Rinsho Meneki Gakkai Kaishi 2015; 38: 390–397.
Das M, Sundell IB, Koka PS . Adult mesenchymal stem cells and their potency in the cell-based therapy. J Stem Cells 2013; 8: 1–16.
De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, Pascual CY, Aller MA, Arias J et al. Immunosuppressive properties of mesenchymal stem cells: advances and applications. Curr Mol Med 2012; 12: 574–591.
Ryu KH, Cho KA, Park HS, Kim JY, Woo SY, Jo I et al. Tonsil-derived mesenchymal stromal cells: evaluation of biologic, immunologic and genetic factors for successful banking. Cytotherapy 2012; 14: 1193–1202.
Park M, Kim YH, Ryu JH, Woo SY, Ryu KH . Immune suppressive effects of tonsil-derived mesenchymal stem cells on mouse bone-marrow-derived dendritic cells. Stem Cells Int 2015; 2015: 106540.
Ryu JH, Park M, Kim BK, Ryu KH, Woo SY . Tonsil-derived mesenchymal stromal cells produce CXCR2-binding chemokines and acquire follicular dendritic cell-like phenotypes under TLR3 stimulation. Cytokine 2015; 73: 225–235.
Pennell LM, Galligan CL, Fish EN . Sex affects immunity. J Autoimmun 2012; 38: J282–J291.
Quintero OL, Amador-Patarroyo MJ, Montoya-Ortiz G, Rojas-Villarraga A, Anaya JM . Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J Autoimmun 2012; 38: J109–J119.
Lee TP, Chiang BL . Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev 2012; 11: A422–A429.
Incorvaia E, Sicouri L, Petersen-Mahrt SK, Schmitz KM . Hormones and AID: balancing immunity and autoimmunity. Autoimmunity 2013; 46: 128–137.
Young NA, Wu LC, Burd CJ, Friedman AK, Kaffenberger BH, Rajaram MV et al. Estrogen modulation of endosome-associated toll-like receptor 8: an IFNalpha-independent mechanism of sex-bias in systemic lupus erythematosus. Clin Immunol 2014; 151: 66–77.
Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG et al. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med 2005; 201: 1375–1383.
Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dörner T et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 2006; 6: 741–750.
Egwuagu CE, Yu CR, Sun L, Wang R . Interleukin 35: Critical regulator of immunity and lymphocyte-mediated diseases. Cytokine Growth Factor Rev 2015; 26: 587–593.
Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM et al. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 2007; 450: 566–569.
Wang RX, Yu CR, Dambuza IM, Mahdi RM, Dolinska MB, Sergeev YV et al. Interleukin-35 induces regulatory B cells that suppress autoimmune disease. Nat Med 2014; 20: 633–641.
Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF . A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity 2008; 28: 639–650.
Iwata Y, Matsushita T, Horikawa M, Dilillo DJ, Yanaba K, Venturi GM et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood 2011; 117: 530–541.
Benedek G, Zhang J, Bodhankar S, Nguyen H, Kent G, Jordan K et al. Estrogen induces multiple regulatory B cell subtypes and promotes M2 microglia and neuroprotection during experimental autoimmune encephalomyelitis. J Neuroimmunol 2016; 293: 45–53.
Bodhankar S, Wang C, Vandenbark AA, Offner H . Estrogen-induced protection against experimental autoimmune encephalomyelitis is abrogated in the absence of B cells. Eur J Immunol 2011; 41: 1165–1175.
Zhang J, Benedek G, Bodhankar S, Lapato A, Vandenbark AA, Offner H . IL-10 producing B cells partially restore E2-mediated protection against EAE in PD-L1 deficient mice. J Neuroimmunol 2015; 285: 129–136.
Joo SY, Cho KA, Jung YJ, Kim HS, Park SY, Choi YB et al. Mesenchymal stromal cells inhibit graft-versus-host disease of mice in a dose-dependent manner. Cytotherapy 2010; 12: 361–370.
Cao W, Cao K, Cao J, Wang Y, Shi Y . Mesenchymal stem cells and adaptive immune responses. Immunol Lett 2015; 168: 147–153.
Glenn JD, Whartenby KA . Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014; 6: 526–539.
Park M, Kim YH, Woo SY, Lee HJ, Yu Y, Kim HS et al. Tonsil-derived mesenchymal stem cells ameliorate CCl4-induced liver fibrosis in mice via autophagy activation. Sci Rep 2015; 5: 8616.
Ryu KH, Kim SY, Kim YR, Woo SY, Sung SH, Kim HS et al. Tonsil-derived mesenchymal stem cells alleviate concanavalin A-induced acute liver injury. Exp Cell Res 2014; 326: 143–154.
Wang D, Huang S, Yuan X, Liang J, Xu R, Yao G et al. The regulation of the Treg/Th17 balance by mesenchymal stem cells in human systemic lupus erythematosus. Cell Mol Immunol, e-pub ahead of print 5 October 2015 doi:10.1038/cmi.2015.89.
Rojas-Villarraga A, Toro CE, Espinosa G, Rodríguez-Velosa Y, Duarte-Rey C, Mantilla RD et al. Factors influencing polyautoimmunity in systemic lupus erythematosus. Autoimmun Rev 2010; 9: 229–232.
Nussinovitch U, Shoenfeld Y . The role of gender and organ specific autoimmunity. Autoimmun Rev 2012; 11: A377–A385.
Park MJ, Kwok SK, Lee SH, Kim EK, Park SH, Cho ML . Adipose tissue-derived mesenchymal stem cells induce expansion of interleukin-10-producing regulatory B cells and ameliorate autoimmunity in a murine model of systemic lupus erythematosus. Cell Transplant 2015; 24: 2367–2377.
Meka RR, Venkatesha SH, Dudics S, Acharya B, Moudgil KD . IL-27-induced modulation of autoimmunity and its therapeutic potential. Autoimmun Rev 2015; 14: 1131–1141.
Shen P, Roch T, Lampropoulou V, O'Connor RA, Stervbo U, Hilgenberg E et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature 2014; 507: 366–370.
Kim N, Cho SG . Overcoming immunoregulatory plasticity of mesenchymal stem cells for accelerated clinical applications. Int J Hematol 2016; 103: 129–137.
Thaunat O, Field AC, Dai J, Louedec L, Patey N, Bloch MF et al. Lymphoid neogenesis in chronic rejection: evidence for a local humoral alloimmune response. Proc Natl Acad Sci USA 2005; 102: 14723–14728.
Thaunat O, Patey N, Morelon E, Michel JB, Nicoletti A . Lymphoid neogenesis in chronic rejection: the murderer is in the house. Curr Opin Immunol 2006; 18: 576–579.
Correa RR, Machado JR, da Silva MV, Helmo FR, Guimarães CS, Rocha LP et al. The importance of C4d in biopsies of kidney transplant recipients. Clin Dev Immunol 2013; 2013: 678180.
Acknowledgements
This research was supported by the Bio & Medical Technology Development Program of the NRF funded by the Korean government (2012M3A9C6049823). In addition, this work was supported by RP-Grant 2016 of Ewha Womans University.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information for this article can be found on the Cellular & Molecular Immunology website
Supplementary information
Rights and permissions
About this article
Cite this article
Cho, KA., Lee, JK., Kim, YH. et al. Mesenchymal stem cells ameliorate B-cell-mediated immune responses and increase IL-10-expressing regulatory B cells in an EBI3-dependent manner. Cell Mol Immunol 14, 895–908 (2017). https://doi.org/10.1038/cmi.2016.59
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.59
Keywords
This article is cited by
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment
Cell Communication and Signaling (2023)
-
Mesenchymal Stem Cell-Derived Exosomes Attenuate TLR7-Mediated Mast Cell Activation
Tissue Engineering and Regenerative Medicine (2022)
-
IL-1β pre-stimulation enhances the therapeutic effects of endometrial regenerative cells on experimental colitis
Stem Cell Research & Therapy (2021)
-
Taming of Covid-19: potential and emerging application of mesenchymal stem cells
Cytotechnology (2021)
-
Old Friends with Unexploited Perspectives: Current Advances in Mesenchymal Stem Cell-Based Therapies in Asthma
Stem Cell Reviews and Reports (2021)