Abstract
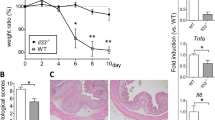
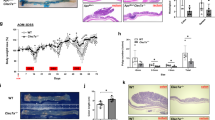
Inflammatory bowel disease (IBD) is an important factor in the induction of colon cancer, but its mechanism is unclear. Colitis and colitis-associated colorectal cancer (CAC) models induced using both dextran sulfate sodium (DSS) and the azoxymethane/DSS protocol were established in wild-type (WT) and CTRP4 transgenic (CTRP4-tg) C57BL6/J mice. Body weight, stool consistency and the presence of blood in the stool were analyzed; tumor quantity, size and histological characteristics were analyzed during the development of CAC. The CTRP4-tg mice exhibited significantly reduced colitis and developed far fewer macroscopic tumors; these tumors were smaller in size, and a majority of the colon tumors in these mice were restricted to the superficial mucosa. Tumors of lower grades were observed in the CTRP4-tg mice. Interleukin-6 was markedly downregulated in the CTRP4-tg mice during CAC tumorigenesis. The phosphorylation of ERK, signal transducer and activator of transcription 3 and Akt in the colon and the proliferation of intestinal epithelial cells were decreased in the CTRP4-tg mice. The injection of recombinant CTRP4 protein significantly reduced the colitis symptoms of the WT mice. CTRP4 plays an important role in inflammation and inflammation-associated colon tumorigenesis, and our research may provide a novel method for the treatment of IBD and CAC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Abraham C, Cho JH . Inflammatory bowel disease. N Engl J Med 2009; 361: 2066–2078.
Kaser A, Zeissig S, Blumberg RS . Inflammatory bowel disease. Annu Rev Immunol 2010; 28: 573–621.
Abraham C, Cho JH . IL-23 and autoimmunity: new insights into the pathogenesis of inflammatory bowel disease. Annu Rev Med 2009; 60: 97–110.
Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN . Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 1994; 35: 1590–1592.
Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 2008; 118: 560–570.
Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009; 15: 103–113.
Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest 2007; 117: 3846–3856.
Li Q, Wang L, Tan W, Peng Z, Luo Y, Zhang Y et al. Identification of C1qTNF-related protein 4 as a potential cytokine that stimulates the STAT3 and NF-kappaB pathways and promotes cell survival in human cancer cells. Cancer Lett 2011; 308: 203–214.
Atreya R, Neurath MF . Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer. Clin Rev Allergy Immunol 2005; 28: 187–196.
Strober W, Fuss I, Mannon P . The fundamental basis of inflammatory bowel disease. J Clin Invest 2007; 117: 514–521.
Becker C, Fantini MC, Schramm C, Lehr HA, Wirtz S, Nikolaev A et al. TGF-beta suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity 2004; 21: 491–501.
Dann SM, Spehlmann ME, Hammond DC, Iimura M, Hase K, Choi LJ et al. IL-6-dependent mucosal protection prevents establishment of a microbial niche for attaching/effacing lesion-forming enteric bacterial pathogens. J Immunol 2008; 180: 6816–6826.
Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med 2000; 6: 583–588.
Heikkila K, Ebrahim S, Lawlor DA . Systematic review of the association between circulating interleukin-6 (IL-6) and cancer. Eur J Cancer 2008; 44: 937–945.
Schaffler A, Buechler C . CTRP family: linking immunity to metabolism. Trends Endocrinol Metab 2012; 23: 194–204.
Wong GW, Wang J, Hug C, Tsao TS, Lodish HF . A family of Acrp30/adiponectin structural and functional paralogs. Proc Natl Acad Sci USA 2004; 101: 10302–10307.
Jeon JH, Kim KY, Kim JH, Baek A, Cho H, Lee YH et al. A novel adipokine CTRP1 stimulates aldosterone production. FASEB J 2008; 22: 1502–1511.
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Revett T, Gimeno R, Lodish HF . Molecular, biochemical and functional characterizations of C1q/TNF family members: adipose-tissue-selective expression patterns, regulation by PPAR-gamma agonist, cysteine-mediated oligomerizations, combinatorial associations and metabolic functions. Biochem J 2008; 416: 161–177.
Kopp A, Bala M, Buechler C, Falk W, Gross P, Neumeier M et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology 2010; 151: 5267–5278.
Compton SA, Cheatham B . CTRP-3: blocking a toll booth to obesity-related inflammation. Endocrinology 2010; 151: 5095–5097.
Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C et al. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J 2009; 23: 241–258.
Byerly MS, Petersen PS, Ramamurthy S, Seldin MM, Lei X, Provost E et al. C1q/TNF-related protein 4 (CTRP4) is a unique secreted protein with two tandem C1q domains that functions in the hypothalamus to modulate food intake and body weight. J Biol Chem 2014; 289: 4055–4069.
Cooper HS, Murthy SN, Shah RS, Sedergran DJ . Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 1993; 69: 238–249.
Stopfer P, Obermeier F, Dunger N, Falk W, Farkas S, Janotta M et al. Blocking lymphotoxin-beta receptor activation diminishes inflammation via reduced mucosal addressin cell adhesion molecule-1 (MAdCAM-1) expression and leucocyte margination in chronic DSS-induced colitis. Clin Exp Immunol 2004; 136: 21–29.
Na D, Ma Z, Luo Y, Li Q, Tan W, Wang L et al. Establishment of adipocytokine CTRP4 transgenic mouse. Chin J Comp Med 2014; 24: 7.
Li B, Alli R, Vogel P, Geiger TL . IL-10 modulates DSS-induced colitis through a macrophage-ROS-NO axis. Mucosal Immunol 2013; 7: 869–878.
Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK et al. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J Clin Invest 2008; 118: 534–544.
Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ et al. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity 2010; 33: 279–288.
Putoczki TL, Thiem S, Loving A, Busuttil RA, Wilson NJ, Ziegler PK et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013; 24: 257–271.
Wang W, Li T, Wang X, Yuan W, Cheng Y, Zhang H et al. FAM19A4 is a novel cytokine ligand of formyl peptide receptor 1 (FPR1) and is able to promote the migration and phagocytosis of macrophages. Cell Mol Immunol 2015; 12: 615–624.
Antonioli L, Blandizzi C, Pacher P, Hasko G . Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer 2013; 13: 842–857.
Grivennikov SI, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Gupta J, del Barco Barrantes I, Igea A, Sakellariou S, Pateras IS, Gorgoulis VG et al. Dual function of p38alpha MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 2014; 25: 17.
Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012; 491: 254–258.
Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009; 15: 91–102.
Kishimoto T . Interleukin-6: from basic science to medicine—40 years in immunology. Annu Rev Immunol 2005; 23: 1–21.
Fu XY . STAT3 in immune responses and inflammatory bowel diseases. Cell Res 2006; 16: 214–219.
Schenk M, Bouchon A, Seibold F, Mueller C . TREM-1—expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest 2007; 117: 3097–3106.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118: 285–296.
Cox JH, Kljavin NM, Ota N, Leonard J, Roose-Girma M, Diehl L et al. Opposing consequences of IL-23 signaling mediated by innate and adaptive cells in chemically induced colitis in mice. Mucosal Immunol 2012; 5: 99–109.
Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118: 285–296.
Arranz A, Doxaki C, Vergadi E, Martinez de la Torre Y, Vaporidi K, Lagoudaki ED et al. Akt1 and Akt2 protein kinases differentially contribute to macrophage polarization. Proc Natl Acad Sci USA 2012; 109: 9517–9522.
Ohashi K, Parker JL, Ouchi N, Higuchi A, Vita JA, Gokce N et al. Adiponectin promotes macrophage polarization toward an anti-inflammatory phenotype. J Biol Chem 2010; 285: 6153–6160.
Lin Y, Yang X, Yue W, Xu X, Li B, Zou L et al. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol 2014; 11: 355–366.
Sarra M, Pallone F, Macdonald TT, Monteleone G . IL-23/IL-17 axis in IBD. Inflamm Bowel Dis 2010; 16: 1808–1813.
Zhao J, Zhang Z, Luan Y, Zou Z, Sun Y, Li Y et al. Pathological functions of interleukin-22 in chronic liver inflammation and fibrosis with hepatitis B virus infection by promoting T helper 17 cell recruitment. Hepatology 2014; 59: 1331–1342.
Acknowledgements
We thank Xiaoyan Qiu for assistance with the H&E staining and histological analysis and Dalong Ma for insightful discussion and suggestions. This work was supported by grants from the National Natural Science Foundation of China (No. 91129707, No. 81172001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Luo, Y., Wu, X., Ma, Z. et al. Expression of the novel adipokine C1qTNF-related protein 4 (CTRP4) suppresses colitis and colitis-associated colorectal cancer in mice. Cell Mol Immunol 13, 688–699 (2016). https://doi.org/10.1038/cmi.2016.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2016.16
Keywords
This article is cited by
-
Association of serum CTRP4 levels with vascular endothelial function in patients with type 2 diabetes mellitus: CTRP4 ameliorating inflammation, proliferation and migration in human umbilical vein endothelial cells
Acta Diabetologica (2024)
-
C1q/TNF-related protein 4 restores leptin sensitivity by downregulating NF-κB signaling and microglial activation
Journal of Neuroinflammation (2021)
-
NLR members in inflammation-associated carcinogenesis
Cellular & Molecular Immunology (2017)
-
CTRP4: a new member of the adipocytokine family
Cellular & Molecular Immunology (2017)