Abstract
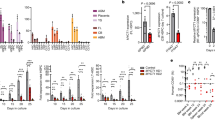
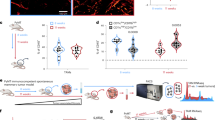
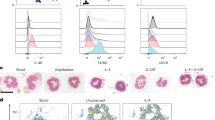
Interferon gamma (IFN-γ) and leukemia inhibitory factor (LIF) are key gestational factors that may differentially affect leukocyte function during gestation. Because IFN-γ induces a pro-inflammatory phenotype in macrophages and because trophoblast cells are principal targets of LIF in the placenta, we investigated whether and how soluble factors from trophoblast cells regulate the effects of IFN-γ on macrophage activation. IFN-γ reduces macrophage motility, but enhances Stat1 activation, pro-inflammatory gene expression and cytotoxic functions. Soluble factors from villous cytotrophoblasts (vCT+LIF cells) and BeWo cells (BW/ST+LIF cells) that were differentiated in the presence of LIF inhibit macrophage Stat1 activation but inversely sustain Stat3 activation in response to IFN-γ. vCT+LIF cells produce soluble factors that induce Stat3 activation; this effect is partially abrogated in the presence of neutralizing anti-interleukin 10 (IL-10) antibodies. Moreover, soluble factors from BW/ST+LIF cells reduce cell proliferation but enhance the migratory responses of monocytes. In addition, these factors reverse the inhibitory effect of IFN-γ on monocyte/macrophage motility. BW/ST+LIF cells also generate IFN-γ-activated macrophages with enhanced IL-10 expression, but reduced tumor-necrosis factor alpha (TNF-α), CD14 and CD40 expression as well as impaired cytotoxic function. Additional assays performed in the presence of neutralizing anti-IL-10 antibodies and exogenous IL-10 demonstrate that reduced macrophage cytotoxicity and proliferation, but increased cell motility result from the ability of trophoblast IL-10 to sustain Stat3 activation and suppress IFN-γ-induced Stat1 activation. These in vitro studies are the first to describe the regulatory role of the LIF–trophoblast–IL-10 axis in the process of macrophage activation in response to pro-inflammatory cytokines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Mosser DM . The many faces of macrophage activation. J Leuk Biol 2003; 73: 209–212.
Gordon S . Alternative activation of macrophages. Nat Rev Immunol 2003; 3: 23–35.
Cohen HB, Mosser DM . Extrinsic and intrinsic control of macrophage inflammatory responses. J Leuk Biol 2013; 94: 913–919.
Mosser DM, Zhang X . Activation of murine macrophages. Curr Protoc Immunol 2008; Chapter 14: Unit 14 2.
Yang Z, Kong B, Mosser DM, Zhang X . TLRs, macrophages, and NK cells: our understandings of their functions in uterus and ovary. Int Immunopharmacol 2011; 11: 1442–1450.
Zhang X, Mosser DM . Macrophage activation by endogenous danger signals. J Pathol 2008; 214: 161–178.
Nagamatsu T, Schust DJ . The contribution of macrophages to normal and pathological pregnancies. Am J Reprod Immunol 2010; 63: 460–471.
Renaud SJ, Graham CH . The role of macrophages in utero–placental interactions during normal and pathological pregnancy. Immunol Invest 2008; 37: 535–564.
Gustafsson C, Mjosberg J, Matussek A, Geffers R, Matthiesen L, Berg G et al. Gene expression profiling of human decidual macrophages: evidence for immunosuppressive phenotype. PLoS ONE 2008; 3: e2078.
Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J . Macrophages at the fetal–maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol 2011; 187: 3671–3682.
Heikkinen J, Mottonen M, Komi J, Alanen A, Lassila O . Phenotypic characterization of human decidual macrophages. Clin Exp Immunol 2003; 131: 498–505.
Wu ZM, Yang H, Li M, Yeh CC, Schatz F, Lockwood CJ et al. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta 2012; 33: 188–194.
Granot I, Gnainsky Y, Dekel N . Endometrial inflammation and effect on implantation improvement and pregnancy outcome. Reproduction 2012; 144: 661–668.
Mor G, Cardenas I, Abrahams V, Guller S . Inflammation and pregnancy: the role of the immune system at the implantation site. Ann NY Acad Sci 2011; 1221: 80–87.
Boehm U, Klamp T, Groot M, Howard JC . Cellular responses to interferon-gamma. Annu Rev Immunol 1997; 15: 749–795.
Murphy SP, Tayade C, Ashkar AA, Hatta K, Zhang J, Croy BA . Interferon gamma in successful pregnancies. Biol Reprod 2009; 80: 848–859.
Clark DA, Chaouat G, Arck PC, Mittruecker HW, Levy GA . Cytokine-dependent abortion in CBA×DBA/2 mice is mediated by the procoagulant fgl2 prothrombinase [correction of prothombinase]. J Immunol 1998; 160: 545–549.
Gendron RL, Nestel FP, Lapp WS, Baines MG . Lipopolysaccharide-induced fetal resorption in mice is associated with the intrauterine production of tumour necrosis factor-alpha. J Reprod Fertil 1990; 90: 395–402.
Murphy SP, Fast LD, Hanna NN, Sharma S . Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol 2005; 175: 4084–4090.
Murphy SP, Hanna NN, Fast LD, Shaw SK, Berg G, Padbury JF et al. Evidence for participation of uterine natural killer cells in the mechanisms responsible for spontaneous preterm labor and delivery. Am J Obstetr Gynecol 2009; 200: 308.e1–308.e9.
Haddad EK, Duclos AJ, Lapp WS, Baines MG . Early embryo loss is associated with the prior expression of macrophage activation markers in the decidua. J Immunol 1997; 158: 4886–4892.
Fest S, Aldo PB, Abrahams VM, Visintin I, Alvero A, Chen R et al. Trophoblast–macrophage interactions: a regulatory network for the protection of pregnancy. Am J Reprod immunol 2007; 57: 55–66.
Dimitriadis E, White CA, Jones RL, Salamonsen LA . Cytokines, chemokines and growth factors in endometrium related to implantation. Hum Reprod Update 2005; 11: 613–630.
Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N et al. Decidual stromal cell response to paracrine signals from the trophoblast: amplification of immune and angiogenic modulators. Biol Reprod 2007; 76: 102–117.
Kimber SJ . Leukaemia inhibitory factor in implantation and uterine biology. Reproduction 2005; 130: 131–145.
Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL et al. Endogenous leukemia inhibitory factor attenuates endotoxin response. Lab Invest 2005; 85: 276–284.
Aisemberg J, Vercelli CA, Bariani MV, Billi SC, Wolfson ML, Franchi AM . Progesterone is essential for protecting against LPS-induced pregnancy loss. LIF as a potential mediator of the anti-inflammatory effect of progesterone. PLoS ONE 2013; 8: e56161.
Szony BJ, Bata-Csorgo Z, Bartfai G, Kemeny L, Dobozy A, Kovacs L . Interleukin-10 receptors are expressed by basement membrane anchored, alpha6) integrin+ cytotrophoblast cells in early human placenta. Mol Hum Reprod 1999; 5: 1059–1065.
Thaxton JE, Sharma S . Interleukin-10: a multi-faceted agent of pregnancy. Am J Reprod Immunol 2010; 63: 482–491.
D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G . Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 1993; 178: 1041–1048.
Wang P, Wu P, Siegel MI, Egan RW, Billah MM . IL-10 inhibits transcription of cytokine genes in human peripheral blood mononuclear cells. J Immunol 1994; 153: 811–816.
Dealtry GB, O'Farrell MK, Fernandez N . The Th2 cytokine environment of the placenta. Int Arch Allergy Immunol 2000; 123: 107–119.
O'Farrell AM, Liu Y, Moore KW, Mui AL . IL-10 inhibits macrophage activation and proliferation by distinct signaling mechanisms: evidence for Stat3-dependent and -independent pathways. EMBO J 1998; 17: 1006–1018.
Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP et al. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma- induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 1999; 93: 1456–1463.
Sharkey AM, King A, Clark DE, Burrows TD, Jokhi PP, Charnock-Jones DS et al. Localization of leukemia inhibitory factor and its receptor in human placenta throughout pregnancy. Biol Reprod 1999; 60: 355–364.
Dufresne M, Dumas G, Asselin E, Carrier C, Pouliot M, Reyes-Moreno C . Pro-inflammatory type-1 and anti-inflammatory type-2 macrophages differentially modulate cell survival and invasion of human bladder carcinoma T24 cells. Mol Immunol 2011; 48: 1556–1567.
Dumas G, Dufresne M, Asselin E, Girouard J, Carrier C, Reyes-Moreno C . CD40 pathway activation reveals dual function for macrophages in human endometrial cancer cell survival and invasion. Cancer Immunol Immunother 2012; 62: 273–283.
Leduc K, Bourassa V, Asselin E, Leclerc P, Lafond J, Reyes-Moreno C . Leukemia inhibitory factor regulates differentiation of trophoblastlike BeWo cells through the activation of JAK/STAT and MAPK3/1 MAP kinase-signaling pathways. Biol Reprod 2012; 86: 54.
Lanoix D, Beghdadi H, Lafond J, Vaillancourt C . Human placental trophoblasts synthesize melatonin and express its receptors. J Pineal Res 2008; 45: 50–60.
Lanoix D, Vaillancourt C . Cell culture media formulation and supplementation affect villous trophoblast HCG release. Placenta 2010; 31: 558–559.
Le Bellego F, Vaillancourt C, Lafond J . Isolation and culture of term human cytotrophoblast cells and in vitro methods for studying human cytotrophoblast cells' calcium uptake. Methods Mol Biol 2009; 550: 73–87.
Orendi K, Gauster M, Moser G, Meiri H, Huppertz B . The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction 2010; 140: 759–766.
Liu F, Soares MJ, Audus KL . Permeability properties of monolayers of the human trophoblast cell line BeWo. Am J Physiol 1997; 273: C1596–C1604.
Wice B, Menton D, Geuze H, Schwartz AL . Modulators of cyclic AMP metabolism induce syncytiotrophoblast formation in vitro. Exp Cell Res 1990; 186: 306–316.
Bennett WA, Lagoo-Deenadayalan S, Brackin MN, Hale E, Cowan BD . Cytokine expression by models of human trophoblast as assessed by a semiquantitative reverse transcription-polymerase chain reaction technique. Am J Reprod Immunol 1996; 36: 285–294.
Bennett WA, Lagoo-Deenadayalan S, Whitworth NS, Brackin MN, Hale E, Cowan BD . Expression and production of interleukin-10 by human trophoblast: relationship to pregnancy immunotolerance. Early Pregnancy 1997; 3: 190–198.
Zhao M, Zhang R, Xu X, Liu Y, Zhang H, Zhai X et al. IL-10 reduces levels of apoptosis in Toxoplasma gondii-infected trophoblasts. PloS ONE 2013; 8: e56455.
Ansa-Addo EA, Lange S, Stratton D, Antwi-Baffour S, Cestari I, Ramirez MI et al. Human plasma membrane-derived vesicles halt proliferation and induce differentiation of THP-1 acute monocytic leukemia cells. J Immunol 2010; 185: 5236–5246.
Auwerx J . The human leukemia cell line, THP-1: a multifacetted model for the study of monocyte-macrophage differentiation. Experientia 1991; 47: 22–31.
Qin Z . The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 2012; 221: 2–11.
Daigneault M, Preston JA, Marriott HM, Whyte MK, Dockrell DH . The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS ONE 2010; 5: 0008668.
Menon MB, Ronkina N, Schwermann J, Kotlyarov A, Gaestel M . Fluorescence-based quantitative scratch wound healing assay demonstrating the role of MAPKAPK-2/3 in fibroblast migration. Cell Motil Cytoskeleton 2009; 66: 1041–1047.
Penton-Rol G, Polentarutti N, Luini W, Borsatti A, Mancinelli R, Sica A et al. Selective inhibition of expression of the chemokine receptor CCR2 in human monocytes by IFN-γ. J Immunol 1998; 160: 3869–3873.
Ashkar AA, Di Santo JP, Croy BA . Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med 2000; 192: 259–270.
Klimp AH, de Vries EG, Scherphof GL, Daemen T . A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol 2002; 44: 143–161.
Bode JG, Ehlting C, Haussinger D . The macrophage response towards LPS and its control through the p38(MAPK)–STAT3 axis. Cell Signal 2012; 24: 1185–1194.
Tamai R, Sugawara S, Takeuchi O, Akira S, Takada H . Synergistic effects of lipopolysaccharide and interferon-gamma in inducing interleukin-8 production in human monocytic THP-1 cells is accompanied by up-regulation of CD14, Toll-like receptor 4, MD-2 and MyD88 expression. J Endotoxin Res 2003; 9: 145–153.
Friebe A, Douglas AJ, Solano E, Blois SM, Hagen E, Klapp BF et al. Neutralization of LPS or blockage of TLR4 signaling prevents stress-triggered fetal loss in murine pregnancy. J Mol Med (Berl) 2011; 89: 689–699.
Reyes-Moreno C, Girouard J, Lapointe R, Darveau A, Mourad W . CD40/CD40 homodimers are required for CD40-induced phosphatidylinositol 3-kinase-dependent expression of B7.2 by human B lymphocytes. J Biol Chem 2004; 279: 7799–7806.
Reyes-Moreno C, Sharif-Askari E, Girouard J, Leveille C, Jundi M, Akoum A et al. Requirement of oxidation-dependent CD40 homodimers for CD154/CD40 bidirectional signaling. J Biol Chem 2007; 282: 19473–19480.
Cella M, Scheidegger D, Palmer-Lehmann K, Lane P, Lanzavecchia A, Alber G . Ligation of CD40 on dendritic cells triggers production of high levels of interleukin-12 and enhances T cell stimulatory capacity: T–T help via APC activation. J Exp Med 1996; 184: 747–752.
Lee SJ, Qin H, Benveniste EN . Simvastatin inhibits IFN-gamma-induced CD40 gene expression by suppressing STAT-1alpha. J Leukoc Biol 2007; 82: 436–447.
Sayama S, Nagamatsu T, Schust DJ, Itaoka N, Ichikawa M, Kawana K et al. Human decidual macrophages suppress IFN-gamma production by T cells through costimulatory B7-H1:PD-1 signaling in early pregnancy. J Reprod Immunol 2013; 100: 109–117.
Co EC, Gormley M, Kapidzic M, Rosen DB, Scott MA, Stolp HA et al. Maternal decidual macrophages inhibit NK cell killing of invasive cytotrophoblasts during human pregnancy. Biol Reprod 2013; 88: 155.
Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD . How cells respond to interferons. Annu Rev Biochem 1998; 67: 227–264.
Hu Y, Hu X, Boumsell L, Ivashkiv LB . IFN-γ and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J Immunol 2008; 180: 8057–8065.
Chen CW, Chang YH, Tsi CJ, Lin WW . Inhibition of IFN-gamma-mediated inducible nitric oxide synthase induction by the peroxisome proliferator-activated receptor gamma agonist, 15-deoxy-delta 12,14-prostaglandin J2, involves inhibition of the upstream Janus kinase/STAT1 signaling pathway. J Immunol 2003; 171: 979–988.
Takaki H, Minoda Y, Koga K, Takaesu G, Yoshimura A, Kobayashi T . TGF-beta1 suppresses IFN-gamma-induced NO production in macrophages by suppressing STAT1 activation and accelerating iNOS protein degradation. Genes Cells 2006; 11: 871–882.
Renaud SJ, Sullivan R, Graham CH . Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta 2009; 30: 313–319.
Hu X, Park-Min KH, Ho HH, Ivashkiv LB . IFN-gamma-primed macrophages exhibit increased CCR2-dependent migration and altered IFN-gamma responses mediated by Stat1. J Immunol 2005; 175: 3637–3647.
Oliveira LJ, McClellan S, Hansen PJ . Differentiation of the endometrial macrophage during pregnancy in the cow. PLoS ONE 2010; 5: e13213.
Kim JS, Kim JG, Moon MY, Jeon CY, Won HY, Kim HJ et al. Transforming growth factor-beta1 regulates macrophage migration via RhoA. Blood 2006; 108: 1821–1829.
Acknowledgements
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC), the Fonds Québécois de la Recherche sur la Nature et les Technologies (FQRNT) and the Réseau Québécois en Reproduction (RQR) to CR-M AD, RD and JH-M were supported by the RQR-CREATE scholarships program. JG holds a postdoctoral fellowship from the Fonds de la Recherche en Santé du Québec (FRSQ). The authors wish to acknowledge Nahla Mrad and Rodrigo Flores Soto for technical assistance in cell signaling and cell motility studies as well as Dr Monique Cadrin and Dr Céline Van Themsche for critical reading of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Cellular & Molecular Immunology's website. (http://www.nature.com/cmi).
Supplementary information
Rights and permissions
About this article
Cite this article
Dallagi, A., Girouard, J., Hamelin-Morrissette, J. et al. The activating effect of IFN-γ on monocytes/macrophages is regulated by the LIF–trophoblast–IL-10 axis via Stat1 inhibition and Stat3 activation. Cell Mol Immunol 12, 326–341 (2015). https://doi.org/10.1038/cmi.2014.50
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2014.50
Keywords
This article is cited by
-
LIF Aggravates Pulpitis by Promoting Inflammatory Response in Macrophages
Inflammation (2024)
-
Epigenomic and transcriptomic analyses reveal differences between low-grade inflammation and severe exhaustion in LPS-challenged murine monocytes
Communications Biology (2022)
-
Colorectal cancer development is affected by the ECM molecule EMILIN-2 hinging on macrophage polarization via the TLR-4/MyD88 pathway
Journal of Experimental & Clinical Cancer Research (2022)
-
Tannic acid alleviates experimental pulmonary fibrosis in mice by inhibiting inflammatory response and fibrotic process
Inflammopharmacology (2020)
-
Interaction of NK Cells, Trophoblast, and Endothelial Cells during Angiogenesis
Bulletin of Experimental Biology and Medicine (2019)