Abstract
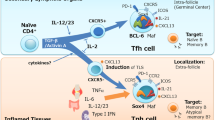
Follicular helper T cells (Tfh) have been referred as a lineage that provides a help for B cells to proliferate and undergo antibody affinity maturation in the germinal center. Evidence has supported that Tfh subset development, like other lineages, is dependent on microenvironment where a particular transcriptional program is initiated. It has been shown that Bcl-6 and IL-21 act as master regulators for the development and function of Tfh cells. Tfh dysregulation is involved in the development of autoimmune pathologies, such as systemic lupus erythematosus, rheumatoid arthritis and other autoimmune diseases. The present review highlights the recent advances in the field of Tfh cells and focus on their development and function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Fazilleau N, Mark L, McHeyzer-Williams LJ, McHeyzer-Williams MG . Follicular helper T cells: lineage and location. Immunity 2009; 30: 324–335.
Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med 2008; 205: 2873–2886.
Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med 2011; 17: 983–988.
Wollenberg I, Agua-Doce A, Hernandez A, Almeida C, Oliveira VG, Faro J et al. Regulation of the germinal center reaction by foxp3+ follicular regulatory T cells. J Immunol 2011; 187: 4553–4560.
Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med 2009; 206: 561–576.
Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum 2010; 62: 234–244.
Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY et al. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat Immunol 2002; 3: 549–557.
Kurata H, Lee HJ, O'Garra A, Arai N . Ectopic expression of activated Stat6 induces the expression of Th2-specific cytokines and transcription factors in developing Th1 cells. Immunity 1999; 11: 677–688.
Zhou X, Wang J, Shi W, Brand DD, Liu Z, Fan H et al. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol 2010; 2: 164–169.
Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA . Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol 2004; 172: 5213–5221.
Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282: 9358–9363.
Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol 2010; 11: 527–534.
Staudt V, Bothur E, Klein M, Lingnau K, Reuter S, Grebe N et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 2010; 33: 192–202.
Breitfeld D, Ohl L, Kremmer E, Ellwart J, Sallusto F, Lipp M et al. Follicular B helper T cells express CXC chemokine receptor 5, localize to B cell follicles, and support immunoglobulin production. J Exp Med 2000; 192: 1545–1452.
Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B . CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med 2000; 192: 1553–1562.
Ansel KM, Ngo VN, Hyman PL, Luther SA, Forster R, Sedgwick JD et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000; 406: 309–314.
Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999; 99: 23–33.
Hardtke S, Ohl L, Forster R . Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 2005; 106: 1924–1931.
Campbell DJ, Kim CH, Butcher EC . Chemokines in the systemic organization of immunity. Immunol Rev 2003; 195: 58–71.
Deenick EK, Ma CS, Brink R, Tangye SG . Regulation of T follicular helper cell formation and function by antigen presenting cells. Curr Opin Immunol 2011; 23: 111–118.
Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L et al. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29: 138–149.
Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L et al. ICOS receptor instructs T follicular helper cell versus effector cell differentiation via induction of the transcriptional repressor Bcl6. Immunity 2011; 34: 932–946.
Good-Jacobson KL, Szumilas CG, Chen L, Sharpe AH, Tomayko MM, Shlomchik MJ . PD-1 regulates germinal center B cell survival and the formation and affinity of long-lived plasma cells. Nat Immunol 2010; 11: 535–542.
Kashiwakuma D, Suto A, Hiramatsu Y, Ikeda K, Takatori H, Suzuki K et al. B and T lymphocyte attenuator suppresses IL-21 production from follicular Th cells and subsequent humoral immune responses. J Immunol 2010; 185: 2730–2736.
Nurieva RI, Chung Y, Martinez GJ, Yang XO, Tanaka S, Matskevitch TD et al. Bcl6 mediates the development of T follicular helper cells. Science 2009; 325: 1001–1005.
Kim CH, Rott LS, Clark-Lewis I, Campbell DJ, Wu L, Butcher EC . Subspecialization of CXCR5+ T cells: B helper activity is focused in a germinal center-localized subset of CXCR5+ T cells. J Exp Med 2001; 193: 1373–1381.
Arnold CN, Campbell DJ, Lipp M, Butcher EC . The germinal center response is impaired in the absence of T cell-expressed CXCR5. Eur J Immunol 2007; 37: 100–109.
Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J Immunol 2005; 175: 7867–7879.
Good KL, Bryant VL, Tangye SG . Kinetics of human B cell behavior and amplification of proliferative responses following stimulation with IL-21. J Immunol 2006; 177: 5236–5247.
King C, Tangye SG, Mackay CR . T follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol 2008; 26: 741–766.
Cannons JL, Tangye SG, Schwartzberg PL . SLAM family receptors and SAP adaptors in immunity. Annu Rev Immunol 2011; 29: 665–705.
Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH et al. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T helper cells and TH-17 cells. Nat Immunol 2009; 10: 167–175.
King IL, Mohrs M . IL-4-producing CD4+ T cells in reactive lymph nodes during helminth infection are T follicular helper cells. J Exp Med 2009; 206: 1001–1007.
Reinhardt RL, Liang HE, Locksley RM . Cytokine-secreting follicular T cells shape the antibody repertoire. Nat Immunol 2009; 10: 385–393.
Zaretsky AG, Taylor JJ, King IL, Marshall FA, Mohrs M, Pearce EJ . T follicular helper cells differentiate from Th2 cells in response to helminth antigens. J Exp Med 2009; 206: 991–999.
Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS et al. T follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-Th1/Th2 effector cells that provide help for B cells. J Immunol 2004; 173: 68–78.
Kim CH, Lim HW, Kim JR, Rott L, Hillsamer P, Butcher EC . Unique gene expression program of human germinal center T helper cells. Blood 2004; 104: 1952–1960.
Rasheed AU, Rahn HP, Sallusto F, Lipp M, Muller G . Follicular B helper T cell activity is confined to CXCR5hiICOShi CD4 T cells and is independent of CD57 expression. Eur J Immunol 2006; 36: 1892–1903.
Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K et al. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150). J Immunol 2010; 185: 190–202.
Tsuji M, Komatsu N, Kawamoto S, Suzuki K, Kanagawa O, Honjo T et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science 2009; 323: 1488–1492.
Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011; 34: 108–121.
McGuire HM, Vogelzang A, Ma CS, Hughes WE, Silveira PA, Tangye SG et al. A subset of interleukin-21+ chemokine receptor CCR9+ T helper cells target accessory organs of the digestive system in autoimmunity. Immunity 2011; 34: 602–615.
Deenick EK, Chan A, Ma CS, Gatto D, Schwartzberg PL, Brink R et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 2010; 33: 241–253.
Ansel KM, McHeyzer-Williams LJ, Ngo VN, McHeyzer-Williams MG, Cyster JG . In vivo-activated CD4 T cells upregulate CXC chemokine receptor 5 and reprogram their response to lymphoid chemokines. J Exp Med 1999; 190: 1123–1134.
Kerfoot SM, Yaari G, Patel JR, Johnson KL, Gonzalez DG, Kleinstein SH et al. Germinal center B cell and T follicular helper cell development initiates in the interfollicular zone. Immunity 2011; 34: 947–960.
Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J et al. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 2010; 32: 253–265.
Johnston RJ, Poholek AC, DiToro D, Yusuf I, Eto D, Barnett B et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 2009; 325: 1006–1010.
Yu D, Rao S, Tsai LM, Lee SK, He Y, Sutcliffe EL et al. The transcriptional repressor Bcl-6 directs T follicular helper cell lineage commitment. Immunity 2009; 31: 457–468.
Kusam S, Toney LM, Sato H, Dent AL . Inhibition of Th2 differentiation and GATA-3 expression by BCL-6. J Immunol 2003; 170: 2435–2441.
Klein U, Dalla-Favera R . Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol 2008; 8: 22–33.
Crotty S . Follicular helper CD4 T cells (TFH). Annu Rev Immunol 2011; 29: 621–663.
Hams E, McCarron MJ, Amu S, Yagita H, Azuma M, Chen L et al. Blockade of b7-h1 (programmed death ligand 1) enhances humoral immunity by positively regulating the generation of T follicular helper cells. J Immunol 2011; 186: 5648–5655.
Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H . Inhibition of follicular T-helper cells by CD8+ regulatory T cells is essential for self tolerance. Nature 2010; 467: 328–332.
Kim HJ, Wang X, Radfar S, Sproule TJ, Roopenian DC, Cantor H . CD8+ T regulatory cells express the Ly49 Class I MHC receptor and are defective in autoimmune prone B6-Yaa mice. Proc Natl Acad Sci USA 2011; 108: 2010–2015.
Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature 2005; 435: 452–458.
Vinuesa CG, Sanz I, Cook MC . Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 2009; 9: 845–857.
Grimaldi CM, Hicks R, Diamond B . B cell selection and susceptibility to autoimmunity. J Immunol 2005; 174: 1775–1781.
Dong W, Zhu P, Wang Y, Wang Z . Follicular helper T cells in systemic lupus erythematosus: a potential therapeutic target. Autoimmun Rev 2011; 10: 299–304.
Hu YL, Metz DP, Chung J, Siu G, Zhang M . B7RP-1 blockade ameliorates autoimmunity through regulation of follicular helper T cells. J Immunol 2009; 182: 1421–1428.
Herber D, Brown TP, Liang S, Young DA, Collins M, Dunussi-Joannopoulos K . IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression. J Immunol 2007; 178: 3822–3830.
Acknowledgements
This work was supported in part by grants from the National Institutes of Health R01 AR 059103, Arthritis Foundation; Wright Foundation; the Outstanding Youth Scientist Investigator Award from National Nature Science Foundation of China (30728007) and the American College of Rheumatology Research and Education's Within Our Reach: Finding a Cure for Rheumatoid Arthritis campaign (all to SGZ), National Nature Science Foundation of China (30972951) (XH) and Le Studium and European FEDER grant support (BR).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Chen, M., Guo, Z., Ju, W. et al. The development and function of follicular helper T cells in immune responses. Cell Mol Immunol 9, 375–379 (2012). https://doi.org/10.1038/cmi.2012.18
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2012.18
Keywords
This article is cited by
-
TSLP promoting B cell proliferation and polarizing follicular helper T cell as a therapeutic target in IgG4-related disease
Journal of Translational Medicine (2022)
-
High salt diet accelerates the progression of murine lupus through dendritic cells via the p38 MAPK and STAT1 signaling pathways
Signal Transduction and Targeted Therapy (2020)
-
A protocol to develop T helper and Treg cells in vivo
Cellular & Molecular Immunology (2017)
-
Distinct phenotypic subpopulations of circulating CD4+CXCR5+ follicular helper T cells in children with active IgA vasculitis
BMC Immunology (2016)
-
Intrathymic Tfh/B Cells Interaction Leads to Ectopic GCs Formation and Anti-AChR Antibody Production: Central Role in Triggering MG Occurrence
Molecular Neurobiology (2016)