Abstract
CNS neurons, such as retinal ganglion cells (RGCs), do not normally regenerate injured axons, but instead undergo apoptotic cell death. Regenerative failure is due to inhibitory factors in the myelin and forming glial scar as well as due to an insufficient intrinsic capability of mature neurons to regrow axons. Nevertheless, RGCs can be transformed into an active regenerative state upon inflammatory stimulation (IS) in the inner eye, for instance by lens injury, enabling these RGCs to survive axotomy and to regenerate axons into the lesioned optic nerve. The beneficial effects of IS are mediated by various factors, including CNTF, LIF and IL-6. Consistently, IS activates various signaling pathways, such as JAK/STAT3 and PI3K/AKT/mTOR, in several retinal cell types. Using a conditional knockdown approach to specifically delete STAT3 in adult RGCs, we investigated the role of STAT3 in IS-induced neuroprotection and axon regeneration. Conditional STAT3 knockdown in RGCs did not affect the survival of RGCs after optic nerve injury compared with controls, but significantly reduced the neuroprotective effects of IS. STAT3 depletion significantly compromised CNTF-stimulated neurite growth in culture and IS-induced transformation of RGCs into an active regenerative state in vivo. As a consequence, IS-mediated axonal regeneration into the injured optic nerve was almost completely abolished in mice with STAT3 depleted in RGCs. In conclusion, STAT3 activation in RGCs is involved in neuroprotection and is a necessary prerequisite for optic nerve regeneration upon IS.
Similar content being viewed by others
Main
Like other neurons of the mammalian central nervous system (CNS), mature retinal ganglion cells (RGCs) are incapable of regenerating damaged axons after injury, but instead undergo apoptotic cell death. Thus, axonal damage due to traumatic optic nerve injury or glaucoma inevitably results in irreversible functional loss.1, 2, 3 Inhibitory factors associated with CNS myelin and the glial scar forming at the injury site are major obstacles for regenerating axons.4, 5, 6 Moreover, an insufficient intrinsic ability of RGCs to regrow injured axons essentially contributes to regenerative failure.7, 8, 9, 10 However, transformation of RGCs into an active regenerative state by inflammatory stimulation (IS) enables these neurons to survive injury and to regrow axons into the inhibitory environment of the lesioned optic nerve. IS can be induced either by lens injury11, 12, 13, 14, 15 or by intravitreal application of crystallins16 or toll-like receptor 2 agonists.17, 18 Astrocyte-derived ciliary neurotrophic factor (CNTF) and leukemia inhibitory factor (LIF) have been identified as essential mediators of the neuroprotective and axon growth stimulating effects of IS.19, 20, 21, 22, 23 Recently, IL-6 has been identified as additional factor contributing to IS, mainly mediating disinhibitory effects toward myelin.24
Application of CNTF, LIF or IL-6 as well as IS activates various signaling pathways in retinal cells in vitro and in vivo. These include the Janus kinase/signal transducers and activators of transcription-3 (JAK/STAT3) and phosphatidylinositide 3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) signaling cascades.24, 25, 26, 27 We have recently shown that inhibition of mTOR neither compromised the initial transformation of RGCs into a regenerative state nor the neuroprotective effects of CNTF or IS.28 Nevertheless, maintenance of basal mTOR activity was required to sustain the regenerative state in RGCs and to overcome myelin- and neurocan-mediated growth inhibition.28 Inhibition of JAK by AG490 reportedly compromised CNTF-mediated neurite growth promotion in culture and in vivo25, 27, 29 as well as the regenerative response after IS.19 These data suggest that JAK signaling is essentially involved in mediating the beneficial effects of IS while other studies came to the opposite conclusion.17, 30, 31 Indeed, inhibition of JAK by AG490 may have also affected downstream signals other than STAT3 such as mitogen activated protein kinase/extracellular signal regulated kinase (MAPK/ERK) or PI3K/AKT-signaling.32 Moreover, other retinal cells besides RGCs may have contributed to the observed effects as cells in the inner nuclear layer also become pSTAT3 positive upon intravitreal application of CNTF or upon IS.18, 19 Hence, the role of STAT3 activation specifically in RGCs during IS-induced neuroprotection and axon regeneration remains elusive. The current study therefore addressed this question taking advantage of adeno-associated virus (AAV2)-mediated conditional STAT3 knockdown in RGCs and demonstrates that activation of STAT3 in RGCs is essential for CNTF-induced neurite growth stimulation in vitro and IS-induced neuroprotection and axonal regeneration in vivo.
Results
Depletion of STAT3 expression in RGCs by AAV2
To elucidate the role of STAT3 in RGCs during IS-induced axonal regeneration, we used a conditional knockdown approach. STAT3-floxed mice were intravitreally injected with AAV2 genetically engineered to express Cre recombinase (AAV2-Cre). Control animals received a virus expressing green fluorescent protein (AAV2-GFP). Consistent with previous reports,33, 34, 35 expression of HA-tagged Cre recombinase was specifically detected in cells of the ganglion cell layer (GCL). A transduction rate of ∼90% for RGCs was estimated, based on co-staining of Cre recombinase with the RGC marker βIII tubulin in retinal flat-mounts (Figure 1a). Injection of either AAV2-Cre or AAV2-GFP did not affect the number of RGCs in the retina after 3 weeks compared with untreated control animals (Figure 1b). As expression of non-phosphorylated STAT3 is stronger in retinal astrocytes and Müller cells than in RGCs and significantly upregulated upon optic nerve injury,19 evaluation of AAV-Cre-mediated STAT3 knockdown in RGCs is difficult. We therefore evaluated the specificity of the STAT3 knockdown for RGCs on retinal sections by examining phosphorylated STAT3 (pSTAT3) upon ON injury. As previously reported, pSTAT3 was not found in the untreated retina, but faintly detected in RGCs (identified by βIII tubulin staining) after optic nerve crush (ONC). Staining markedly increased in RGCs, in cells of the fiber layer (most likely astrocytes) and in cells of the inner nuclear layer (INL) after ONC+IS (Figure 1c). However, pSTAT3 activation was almost completely absent in RGCs after AAV2-Cre injection, but unchanged in other retinal cells in the INL and fiber layer (Figure 1c). Quantification of western blots revealed a 78% decrease of phosphorylated STAT3 protein in lysates from AAV2-Cre-injected mice after ONC+IS compared with control animals (Figures 1d and e), demonstrating that AAV2-Cre efficiently depleted STAT3 expression/activation in RGCs of floxed mice.
AAV2-mediated conditional STAT3 depletion in RGCs. (a) Retinal flat-mount from a STAT3-floxed mouse 2 weeks after intravitreal application of AAV2-Cre. HA-tagged Cre recombinase (cre, red) was detected in the nuclei of ∼90% of βIII tubulin-positive RGCs (green) using an anti-HA-antibody. Scale bar: 50 μm. (b) Quantification of βIII tubulin-positive RGCs per mm2 in flat-mounted retinae 3 weeks after intravitreal injection of either AAV2-GFP (control) or AAV2-Cre. Virus-mediated knockdown of STAT3 (cre) did not affect the survival of uninjured RGCs compared with AAV2-GFP-treated control retinae (gfp). Values represent the mean of three retinae per group. (c) Immunohistochemical staining of cross sections of untreated control retinae (con) and retinae 5 days after optic nerve crush (onc) or onc+inflammatory stimulation (onc/is) from either AAV2-GFP- (gfp) or AAV2-Cre- (cre) injected mice with antibodies against phosphorylated STAT3 (pSTAT3, red) and βIII tubulin (tubulin, green). Virus-mediated STAT3 knockdown reduced IS-induced activation of STAT3 specifically in RGCs, but not in cells of the fiber and inner nuclear layer. Scale bar: 50 μm; GCL, ganglion cell layer; INL, inner nuclear layer. (d) Western blot analysis of retinal lysates from animals treated as described in c with an antibody against phosphorylated STAT3 (pSTAT3). Tubulin served as loading control. Activation of STAT3 strongly increased after ONC+IS compared with untreated (con) and optic nerve crushed (ONC) AAV2-GFP control mice (gfp). Virus-mediated STAT3 knockdown (cre) strongly diminished STAT3 phosphorylation upon ONC+IS. (e) Photometric quantification of western blots as in d. Virus-mediated STAT3 knockdown (cre) reduced STAT3 activation by 78%. Values represent the mean of four independent experiments. Treatment effects: ***P<0.001
STAT3 knockdown compromises CNTF-mediated neurite growth in culture
CNTF-stimulated neurite growth of dissociated, mature RGCs in mixed cultures is compromised by AG490, a potent JAK inhibitor.19, 28 To test whether, downstream of CNTF, STAT3 phoshorylation is required for neurite growth stimulation in RGCs rather than in other retinal cells, retinal cells were cultured in the absence or presence of CNTF 2 weeks after the intravitreal application of either AAV2-Cre or control AAV2-GFP. As reported previously,28 CNTF significantly increased the average neurite length in cultures derived from control animals (AAV2-GFP) compared with vehicle-treated controls (Figures 2a and b). Neurite growth was comparable in vehicle-treated cultures from AAV2-Cre- and AAV2-GFP-treated animals, suggesting that STAT3 knockdown did not affect basal neurite elongation per se. In contrast, CNTF-induced neurite growth was markedly reduced in retinal cultures from AAV2-Cre animals compared with cultures from AAV2-GFP-treated mice (Figures 2a and b), demonstrating that CNTF-mediated neurite extension depends on STAT3 expression/activation in RGCs. RGC survival was not affected by either treatment after 3 days in culture (Figure 2c).
STAT3 depletion in RGCs compromises the IS-induced switch into a regenerative state. (a) βIII tubulin-positive RGCs of STAT3-floxed mice either exposed to vehicle (con) or CNTF (200 ng/ml) after 3 days in culture. Animals were intravitreally injected with either AAV2-GFP or AAV2-Cre 14 days prior to preparing cultures. Scale bar: 50 μm. (b) Quantification of neurite length per RGC in retinal cultures as described in a. Values represent the mean of 12 wells from three independent retinae per group. Depletion of STAT3 in RGCs significantly compromised CNTF-induced neurite growth. (c) Quantification of RGCs per well in cultures as described in a. STAT3 knockdown did not affect the survival of mature RGCs in these cultures. (d) In vivo pre-conditioned, βIII tubulin-positive RGCs after 24 h in culture. Pre-treatment: STAT3-floxed mice received intravitreal injections of either AAV2-GFP (gfp) or AAV2-Cre (cre). Two weeks later, animals were subjected either to optic nerve crush (onc) or onc+inflammatory stimulation (onc/is). Retinal cell cultures were prepared 5 days thereafter. Scale bar: 50 μm. (e) Quantification of neurite length per cultured RGC from mice treated as described in d. Values represent the mean of 16 wells from four independent retinae per group. STAT3 depletion significantly compromised IS-induced neurite growth. (f) Quantification of RGCs per well in retinal cultures as described in d. Virus-mediated STAT3 depletion did not affect RGC survival in retinal cell cultures. (g, h) Quantitative real-time PCR: retinal Gap43 (g) and Sprr1a (h) expression was quantified relative to GAPDH expression in animals as described in d. Values represent the mean of four retinae per group. ONC- and IS-induced upregulation of GAP43 and Sprr1a expression was impeded upon STAT3 depletion. (i) Western blot analysis of retinal lysates from animals treated as described in d using antibodies against GAP43, CNTF and pSTAT3. Tubulin served as loading control. Expression of GAP43 and CNTF strongly increased after ONC+IS compared with untreated AAV2-GFP control mice (gfp). IS-induced upregulation of GAP43 expression was compromised upon STAT3 depletion (cre), whereas the increase in CNTF expression was not affected. Treatment effects: n.s., non-significant; **P<0.01; ***P<0.001
STAT3 knockdown impedes IS-induced transformation of RGCs into a regenerative state
IS reportedly transforms injured RGCs into an active regenerative state, indicated by the expression of regeneration-associated genes and spontaneous neurite outgrowth of cultured RGCs after prior in vivo treatment.19 However, in the course of IS, STAT3 is activated in RGCs as well as in cells of the fiber and inner nuclear layer (Figure 1c and Muller et al.19 and Hauk et al.18), raising the question whether STAT3 expression/activation in RGCs or in other retinal cells is required for these IS-induced effects. To address this question, STAT3-floxed mice were intravitreally injected with either AAV2-Cre or AAV2-GFP and 2 weeks later subjected to either ONC or ONC+IS. Five days after surgery (before onset of ONC-induced apoptotic cell death), dissociated retinal cell cultures were prepared to determine the average length of spontaneously regenerating neurites, which correlates with the regenerative state of RGCs.19, 22, 36 Consistent with previous reports, spontaneous neurite growth of RGCs from AAV2-GFP-treated control mice was markedly increased upon IS compared with ONC alone (Figures 2d and e). In comparison, AAV2-Cre-induced knockdown of STAT3 in RGCs resulted in markedly reduced neurite growth both after ONC alone and after ONC+IS (Figures 2d and e). Therefore, the level of STAT3 activation in RGCs (none in AAV2-Cre/ONC, minor in AAV2-GFP/ONC, slight in AAV2-Cre/ONC+IS and marked in AAV2-GFP/ONC+IS) correlates well with RGC neurite growth ability. RGC survival was not affected by either treatment in these cultures (Figure 2f).
An active regenerative state of RGCs is associated with increased expression of growth-associated protein 43 (gap43) and small proline-rich protein 1A (sprr1a).37, 38 Consistently, gap43 and sprr1a mRNA levels were elevated upon ONC and even further after ONC+IS compared with untreated controls in AAV2-GFP-injected mice, as determined by quantitative real-time PCR (Figures 2g and h). However, gap43 and sprr1a expression were significantly reduced after ONC alone and after ONC+IS in STAT3-knockdown animals compared with AAV2-GFP treated controls, indicating impaired transformation of RGCs into an active regenerative state. Comparable results were obtained by western blot analysis, as IS-induced GAP43 protein expression in AAV2-GFP-treated retinae was reduced in AAV2-Cre animals (Figure 2i). In contrast, IS-induced CNTF expression was not influenced by AAV2-Cre-mediated STAT3 knockdown, as it occurs upstream of STAT3 activation. Taken together, these data indicate that STAT3 expression/activation in RGCs is essential for IS-induced transformation of these neurons into an active regenerative state.
STAT3 knockdown compromises IS-induced neuroprotection and axonal regeneration in vivo
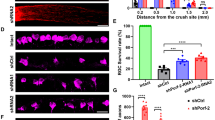
We next investigated whether STAT3 expression/activation in RGCs is required for IS-induced neuroprotection and optic nerve regeneration. To this end, STAT3-floxed mice received intravitreal injections of either AAV2-Cre or AAV2-GFP and were subjected to ONC or ONC+IS 2 weeks later. Two weeks after optic nerve surgery, retinae were isolated and the numbers of surviving RGCs were quantified (Figures 3a and b). Consistent with previous reports,11, 22, 28 ONC significantly reduced the number of surviving RGCs of AAV2-GFP-and AAV2-Cre-injected animals to a similar extent (Figures 3a and b) and IS induced neuroprotection in AAV2-GFP-treated control animals. However, STAT3 depletion moderately, but significantly reduced IS-mediated RGC survival compared with AAV2-GFP control animals (Figures 3a and b), suggesting a partial contribution of STAT3 activation to IS-induced neuroprotection.
STAT3 depletion impairs IS-induced neuroprotection. (a) Immunohistochemical staining of surviving RGCs in retinal flat-mounts 14 days after optic nerve crush (onc) or onc+inflammatory stimulation (onc/is) using an antibody against βIII tubulin. Scale bar: 50 μm. Animals were intravitreally injected with either AAV2-GFP (gfp) or AAV2-Cre (cre) 2 weeks before surgery. (b) Quantification of surviving RGCs per mm2 in retinal flat-mounts of animals treated as described in a. Conditional STAT3 knockdown partially reduced the neuroprotective effect of IS. Treatment effects: n.s., non-significant; *P<0.05; ***P<0.001. Values represent the mean of at least five retinae per treatment
In addition, we analyzed IS-mediated axonal regeneration in vivo. To this end, longitudinal optic nerve sections were stained with an anti-GAP43 antibody and regenerating axons were quantified at various distances beyond the lesion site. As previously reported, AAV2-GFP control mice showed almost no regeneration 14 days after ONC, while IS promoted pronounced axonal growth past the injury site (Figures 4a and b).16, 19, 28 In contrast, STAT3 depletion in RGCs strikingly reduced IS-induced regeneration into the injured optic nerve. The number of axons growing 0.5, 1 or 1.5 mm beyond the lesion site was reduced by ∼80% compared with AAV2-GFP-treated control mice (Figures 4a and b), suggesting that STAT3 expression in RGCs is essential for IS-mediated axonal regeneration in vivo.
STAT3 depletion in RGCs compromises IS-stimulated axon regeneration in the optic nerve. (a) Regenerating axons crossing the lesion site (asterisk) in longitudinal sections of optic nerves visualized with an antibody against GAP43. Scale bar: 100 μm. Animals were pre-treated by intravitreal injection of either AAV2-GFP or AAV2-Cre 2 weeks before optic nerve crush (onc) or onc+inflammatory stimulation (onc/is). Animals were killed 2 weeks later and optic nerves were dissected. (b) Quantification of regenerating axons at 0.5, 1, 1.5 and 2 mm beyond the lesion site in ONC/IS-treated animals injected either with AAV2-GFP (gfp) or AAV2-Cre (cre). STAT3 depletion in RGCs significantly compromised IS-induced axonal regeneration. Treatment effects: ***P<0.001. Values represent the mean of five animals per treatment
Discussion
We and others have previously demonstrated that CNTF- and IS-induced transformation of RGCs into an active regenerative state is associated with phosphorylation and nuclear localization of STAT3 in these neurons, but also in other retinal cells.19, 22, 25, 39 Intravitreal injection of the JAK inhibitor AG490, which partially blocked STAT3 phosphorylation in the inner retina, reportedly compromised CNTF- and, to some extent, IS-mediated axon regeneration.19, 25, 27 Although these data suggest an important role of JAK signaling, it remained elusive whether subsequent neuronal STAT3 activation is required for the transformation of RGCs into a regenerative state or whether AG490 injection may have indirectly compromised regeneration by affecting other cells. Moreover, JAKs also activate and interfere with several other pathways, for example MAPK/ERK- or PI3K/AKT-signaling,32 and therefore AG490 could have impaired the regenerative response by affecting these pathways. In fact, other studies proposed that neither JAK/STAT3 signaling nor CNTF are involved in mediating the beneficial effects of IS.30, 40
To specifically investigate the role of neuronal STAT3 in IS-mediated neuroprotection and axon regeneration, we used an AAV2-Cre recombinase-mediated conditional knockdown approach to deplete STAT3 in mature RGCs. AAV2 is highly neurotropic,41, 42 and intravitreal injection of this virus serotype almost specifically transduces adult RGCs.37, 43, 44, 45 Consistent with previous studies in mice, AAV2-Cre transduction rates were ∼90% in the current study,33, 34, 35 allowing an efficient ablation of STAT3 expression/activation in RGCs as determined by immunohistochemical and western blot analysis. Using this approach, we found that CNTF-stimulated neurite growth was compromised in STAT3-depleted RGCs in culture, whereas basal growth without CNTF stimulation was unaffected. These data therefore demonstrate that STAT3 activation in RGCs has an important role in mediating CNTF-induced neurite growth. Similarly, IS-mediated transformation of RGCs into a regenerative state was strongly compromised upon STAT3 depletion in RGCs in vivo. This was demonstrated among others by the impaired upregulation of regeneration-associated genes, such as gap43 and sprr1a, upon STAT3 depletion. In addition, spontaneous neurite outgrowth in culture after prior IS was reduced, reflecting the diminished regenerative state of STAT3-depleted RGCs. Most strikingly, IS-induced regeneration into the crushed optic nerve in vivo was almost completely abolished by STAT3 depletion, underlining the importance of STAT3 activation in RGCs for the onset of IS-induced regeneration. Nevertheless, other signaling pathways likely contribute to the process of axonal regeneration. For example, CNTF and IS are known to activate the PI3K/AKT cascade. The importance of AKT activation for the initiation of axonal growth has previously been demonstrated,10, 25 whereas mTOR activity is rather required to maintain the regenerative state.28 Similarly, PTEN deletion, which increases PI3K/AKT-signaling, promotes axonal regeneration upon optic nerve injury.45
Consistent with our data in the CNS, STAT3 activation and/or retrograde transport is reportedly required for the initiation, but not the perpetuation of axonal regeneration in peripheral DRG neurons.46, 47, 48, 49 Moreover, expression of constitutively active STAT3 slightly promoted neurite growth of postnatal neurons.50 Whether STAT3 activation is also necessary at later stages in RGC regeneration, for instance to maintain the regenerative state as previously shown for mTOR activity,28 could not be addressed in our study. Nevertheless, knockdown of the suppressor of cytokine signaling 3 (SOCS3), which is a direct target of STAT3-induced expression and itself acts as a negative feedback regulator of STAT3, has been shown to promote axonal regeneration into the optic nerve, which is likely at least partially mediated by disinhibition of STAT3 activity.34, 35 In accordance with this interpretation, axonal regeneration upon SOCS3 deletion is further increased after CNTF application.3435 On the other hand, SOCS3 overexpression in DRG neurons or RGCs reportedly compromised axon regeneration by blocking STAT3 activity.51, 52
IS confers neuroprotection by increasing the number of RGCs surviving after ONC.12, 13, 14 In the current study, we found that STAT3 activation in RGCs partially contributes to IS-induced neuroprotection as the number of surviving RGCs was reduced in STAT3-depleted retinae. This result is in agreement with studies reporting reduced facial motoneuron survival53 and increased apoptosis of sensory neurons54 upon STAT3 gene deletion. STAT3 activation in neurons also correlates with survival in animal models of transient focal ischemia.55 Interestingly, neuroprotection was not affected in animals without IS treatment in our study, suggesting that phosphorylated STAT3 rather than STAT3 expression per se is involved in mediating neuroprotection. However, other signaling pathways are likely involved in this process as the neuroprotective effect of IS was only partially reduced upon STAT3 knockdown. It has previously been shown that PTEN depletion, which activates PI3K/AKT-signaling, mediates RGC survival after optic nerve injury.33 Similarly, intravitreal administration of BDNF induced phosphorylation of AKT, leading to profound neuroprotection of axotomized RGCs.56 Consistently, CNTF and IS also activate PI3K/AKT-signaling, which likely contributes to IS-mediated neuroprotection, explaining the merely partial neuroprotection upon STAT3 knockdown.25, 26, 27, 28
In conclusion, the current study demonstrates that expression and activation of neuronal STAT3 are essentially involved in switching mature RGCs into a regenerative state and contribute to neuroprotection upon IS. Moreover, the current study provides further evidence that glial-derived CNTF and LIF are key factors mediating the transformation of RGCs into a regenerative state upon IS.
Materials and methods
Optic nerve crush and IS
Adult, 2- to 3-month-old, homozygous STAT3-floxed mice (STAT3f/f) on C57BL/6j background were used for all experiments.57 All mice were housed under the same conditions for at least 10 days before being used in experiments. Mice were maintained on a 12 h light/dark cycle with ad libitum access to food and water. All experimental procedures were approved by the local animal care committee in Recklinghausen and conducted in compliance with federal and state guidelines for animal experiments in Germany. Mice received 2 μl intravitreal injections of either AAV2-Cre or AAV2-GFP 2 weeks before further experiments. For surgery, animals were anesthetized by intraperitoneal injection of ketamine (60–80 mg/kg) and xylazine (10–15 mg/kg). The left optic nerve was intraorbitally crushed ∼1 mm behind the eye for 10 s using jeweler's forceps (Hermle, Germany), as described previously).2225 For IS, the lens capsule was retrolentally punctured and 2 μl of (S)-[2,3-Bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH (Pam3Cys dissolved in PBS (1 μg/μl), EMC Microcollections, Germany) were intravitreally injected immediately following optic nerve crush as described recently.22, 24, 28 Five days after surgery, retinae were removed to either prepare protein lysates (n=3 per group), extract RNA (n=4 per group) or dissociate cells for culture experiments (n=6–8 per group). To quantify in vivo axonal regeneration, eyes were isolated with optic nerves attached and prepared for histology 14 days after surgery (n=5–6 per group).
Preparation of AAV2
For AAV2 production, we used the pAAV-MCS plasmid (Stratagene, La Jolla, CA, USA) carrying the cDNA for Cre-HA (kindly provided by Dr. Zhigang He)33 or GFP downstream of the CMV promoter. For recombinant virus generation, AAV-293 cells (Stratagene) were co-transfected with pAAV-RC (Stratagene) encoding the AAV genes rep and cap and the helper plasmid (Stratagene) encoding E24, E4 and VA. Purification of virus particles was performed as described previously.33, 58 Mainly RGCs are transduced upon the intravitreal injection of AAV237, 43, 44, 45 as this virus serotype is highly neurotropic,32, 33 and RGCs are the first neurons to be encountered by the virus.
Dissociated retinal cell cultures
Dissociated retinal cell cultures were prepared as detailed previously.36 In brief, tissue culture plates (four-well-plates; Nunc, Germany) were coated with poly-D-lysine (0.1 mg/ml, molecular weight 300 000 Da) (Sigma, Saint Louis, MO, USA), rinsed with distilled water and air-dried. Wells were then coated with laminin (20 μg/ml, Sigma). To prepare low-density retinal cell cultures, mice, which were either treated with AAV2-Cre/AAV2-GFP for 2 weeks or with AAV2-Cre/AAV2-GFP for 2 weeks plus ONC/ONC+IS for 5 days, were killed by cervical dislocation. Retinae were rapidly dissected from the eyecups and incubated at 37 °C for 30 min in a digestion solution containing papain (10 U/ml, Worthington) and L-cysteine (0.3 μg/ml, Sigma) in Dulbecco's Modified Eagle medium (DMEM, Invitrogen, Carlsbad, CA, USA). They were then rinsed with DMEM and triturated in 2 ml DMEM. To remove cell fragments and factors released from the cells, the cell suspension of one retina was immediately adjusted to a volume of 50 ml with DMEM. Cells were centrifuged for 5 min at 500 × g, and the pellet was carefully re-suspended in 1.5-ml DMEM containing B27 supplement (1 : 50) (Gibco, Carlsbad, CA, USA) and penicillin/streptomycin (0.2 mg/ml) (Biochrom, Cambridge, UK). Dissociated cells were passed through a cell strainer (40 μm) (Falcon, Franklin Lakes, NJ, USA), and 300 μl cell suspension was added to each well. For experiments evaluating the effects of CNTF on neurite outgrowth, 200 ng/ml CNTF (ABD Serotec, Oxford, UK) was added to the culture medium. Neurite growth was determined after 72 h in culture for untreated retinae and after 24 h in culture for primed retinae, respectively. Cells were fixed in 4% paraformaldehyde (PFA) solution in PBS for 25 min and then in 100% methanol (Sigma) for 10 min. RGCs were specifically stained with an antibody against βIII tubulin (clone TUJ-1, 1 : 2000, Covance, NJ, USA). RGCs with regenerated neurites were photographed under a fluorescent microscope ( × 200) and neurite length was determined using ImageJ software. Furthermore, the total number of βIII tubulin-positive RGCs with an intact nucleus (DAPI staining) was quantified per well to evaluate potential neurotoxic treatment effects. Mean neurite length per RGC was calculated by dividing the sum of the neurite length per well by the total number of RGCs per well. Four wells were evaluated per experimental condition, and each experiment was repeated at least three times. Data are given as the mean±S.E.M. of replicate wells. The significance of intergroup difference was evaluated using one-way analysis of variance (ANOVA), followed by corrections for multiple post hoc tests (Bonferroni–Holm, Tukey). The investigator was blinded to the arrangement and identity of the cultures to prevent the introduction of bias to the analysis.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from mouse retinae using the RNeasy kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Retina-derived RNA (40 ng) was reverse transcribed using the superscript II kit (Invitrogen). Quantification of GAP43 and GAPDH expressions was performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and QuantiTect primers (MmGAP43_1_SG, Mm_Gapdh_3_SG; Mm_Sprr1a_2_SG, QuantiTect Primer Assay, Qiagen) on an Applied Biosystems 7500 real-time PCR system. Retina-derived cDNA was amplified during 50 cycles according to the manufacturer’s protocol. All reactions were performed in duplicate, and at least four independent samples (from different eyes) were analyzed per experimental group. The specificity of the PCR product was determined and verified with the dissociation curve analysis feature.
Immunohistochemistry
Animals were anesthetized and perfused through the heart with cold PBS followed by 4% PFA. Eyes with optic nerve segments attached were removed from the connective tissue, post-fixed for several hours in a 4% PFA, transferred to 30% sucrose overnight (4 °C) and embedded in Tissue-Tek (Sakura, Alphen aan den Rijn, Netherlands). Frozen sections (14 μm) were cut longitudinally on a cryostat, thaw-mounted onto glass slides (Superfrost plus, Fisher, Pittsburgh, PA, USA) and stored at −80 °C until further use. A custom-made antibody against GAP43 (1 : 1000, Invitrogen) and a monoclonal antibody against βIII tubulin (clone: TUJ-1, 1 : 1000, Covance) were used to visualize regenerating axons and RGCs. Cre recombinase was visualized using a polyclonal antibody against the HA-tag (1 : 500) (Sigma). Phosphorylated STAT3 (pSTAT3) was detected with a polyclonal antibody (Tyr707, 1 : 200) (Cell Signaling Technology, Danvers, MA, USA). Secondary antibodies included anti-mouse, anti-rabbit and anti-goat IgG antibodies conjugated to Alexa Fluor 488 or Alexa Fluor 594 (1 : 1000) (Molecular Probes, Carlsbad, CA, USA). Fluorescent sections were embedded in Mowiol and analyzed under a fluorescent microscope (Zeiss, Jena, Germany).
Quantification of regenerating axons in the optic nerve
Axon regeneration was quantified as described previously.19, 22 In brief, the number of GAP43- and βIII tubulin-positive axons extending ≥0.5, ≥1.0, ≥1.5 and≥2.0 mm from the injury site were counted under × 400 magnification in 6–8 sections per individual treatment. These values were normalized to the cross-sectional area of the optic nerve and extrapolated to the whole optic nerve. The significances of intergroup differences were evaluated using a one-way ANOVA test, followed by the Holm-Sidak post hoc test. Each experimental group included at least six mice.
Quantification of RGCs in retinal flat-mounts
For quantification of surviving RGCs per mm2, retinal flat-mounts were stained with an antibody against βIII-tubulin (clone: TUJ-1, Covance) (1 : 1000). Retinae were divided into four quadrants. In each quadrant 4–5 independent fields were sampled, proceeding from the center to the periphery. The average number of βIII-tubulin-positive RGCs per field was determined and divided by the area of the field. Values were averaged per retina, averaged across all similarly treated animals to obtain a group mean and S.E. of the mean determined. At least 5–7 retinae per case were analyzed. The significances of intergroup differences were evaluated using a two-way analysis of variance (ANOVA) test, followed by corrections for multiple post hoc tests (Bonferroni–Holm, Tukey).
Western blot assays
For retinal lysate preparation, mice were killed, eyes enucleated and retinae dissected. Isolated retinae were homogenized in lysis buffer (20 mM Tris/HCl pH 7.5, 10 mM KCl, 250 mM sucrose, 10 mM NaF, 1 mM DTT, 0.1 mM Na3VO4, 1% Triton X-100, 0.1% SDS) with 1/100 protease inhibitor (Calbiochem, Darmstadt, Germany), and the lysate was cleared by centrifugation at 5000 r.p.m. for 10 min at 4 °C. Proteins were separated by 10% sodium-dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), according to the standard protocols (Bio-Rad, Hercules, CA, USA) and transferred to nitrocellulose membranes (Amersham, Buckinghamshire, UK). Blots were blocked either in 5% dried milk or in 2% ECL advance blocking agent in Tris-buffered saline containing 1% Tween-20 (TBS-T). They were then processed for immunostaining with either a polyclonal antiserum against phospho-STAT3 (Tyr705, 1 : 5000) (Cell Signaling), a monoclonal antibody against βIII tubulin (clone TUJ-1,1 : 1000) (Covance), a polyclonal antibody against rat CNTF (1 : 5000) (Serotec) or a custom-made antibody against Gap43 (1 : 1000) (Invitrogen) at 4 °C overnight. Bound antibodies were visualized with anti-rabbit, anti-goat/sheep or anti-mouse immunoglobulin G (IgG), secondary antibodies conjugated to horseradish peroxidase (1 : 80 000) (Sigma). Antigen–antibody complexes were detected by enhanced chemiluminescence (ECL) or ECL advance (Amersham).
Abbreviations
- AAV:
-
adeno-associated virus
- AKT:
-
protein kinase B
- CNS:
-
central nervous system
- CNTF:
-
ciliary neurotrophic factor
- Cre:
-
Cre recombinase
- DMEM:
-
Dulbecco's Modified Eagle medium
- GAP43:
-
growth-associated protein 43
- GCL:
-
ganglion cell layer
- GFP:
-
green fluorescent protein
- INL:
-
inner nuclear layer
- IS:
-
inflammatory stimulation
- JAK:
-
Janus kinase
- LIF:
-
leukemia inhibitory factor
- mTOR:
-
mammalian target of rapamycin
- ON:
-
optic nerve
- ONC:
-
optic nerve crush
- Pam3Cys:
-
(S)-[2,3-Bis(palmitoyloxy)-(2-RS)-propyl]-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4-OH
- PFA:
-
paraformaldehyde
- PI3K:
-
phosphatidylinositide 3-kinase
- pSTAT3:
-
phosphorylated STAT3
- RGCs:
-
retinal ganglion cells
- SOCS3:
-
suppressor of cytokine signaling 3
- sprr1a:
-
small proline-rich protein 1A
- STAT3:
-
signal transducers and activators of transcription-3
References
Diekmann H, Fischer D . Glaucoma and optic nerve repair. Cell Tissue Res 2013; 353: 327–337.
Wohl SG, Schmeer CW, Witte OW, Isenmann S . Proliferative response of microglia and macrophages in the adult mouse eye after optic nerve lesion. Invest Ophthalmol Vis Sci 2010; 51: 2686–2696.
Berry M, Ahmed Z, Lorber B, Douglas M, Logan A . Regeneration of axons in the visual system. Restor Neurol Neurosci 2008; 26: 147–174.
Schwab ME, Kapfhammer JP, Bandtlow CE . Inhibitors of neurite growth. Ann Rev Neurosci 1993; 16: 565–595.
Silver J, Miller JH . Regeneration beyond the glial scar. Nat Rev Neurosci 2004; 5: 146–156.
Yiu G, He Z . Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 2006; 7: 617–627.
Goldberg JL, Espinosa JS, Xu Y, Davidson N, Kovacs GT, Barres BA . Retinal ganglion cells do not extend axons by default: promotion by neurotrophic signaling and electrical activity. Neuron 2002; 33: 689–702.
Goldberg JL . Intrinsic neuronal regulation of axon and dendrite growth. Curr Opin Neurobiol 2004; 14: 551–557.
Liu K, Tedeschi A, Park KK, He Z . Neuronal intrinsic mechanisms of axon regeneration. Ann Rev Neurosci 2011; 34: 131–152.
Fischer D, Leibinger M . Promoting optic nerve regeneration. Prog Retin Eye Res 2012; 31: 688–701.
Lorber B, Berry M, Logan A . Lens injury stimulates adult mouse retinal ganglion cell axon regeneration via both macrophage- and lens-derived factors. Eur J Neurosci 2005; 21: 2029–2034.
Fischer D, Heiduschka P, Thanos S . Lens-injury-stimulated axonal regeneration throughout the optic pathway of adult rats. Exp Neurol 2001; 172: 257–272.
Fischer D, Pavlidis M, Thanos S . Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci 2000; 41: 3943–3954.
Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI . Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci 2000; 20: 4615–4626.
Pernet V, Di Polo A . Synergistic action of brain-derived neurotrophic factor and lens injury promotes retinal ganglion cell survival, but leads to optic nerve dystrophy in vivo. Brain 2006; 129 (Pt 4): 1014–1026.
Fischer D, Hauk TG, Muller A, Thanos S . Crystallins of the beta/gamma-superfamily mimic the effects of lens injury and promote axon regeneration. Mol Cell Neurosci 2008; 37: 471–479.
Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR et al. Macrophage-derived factors stimulate optic nerve regeneration. J Neurosci 2003; 23: 2284–2293.
Hauk TG, Leibinger M, Muller A, Andreadaki N, Knippschild U, Fischer D . Intravitreal application of the Toll-like receptor 2 agonist Pam3Cys stimulates axon regeneration in the mature optic nerve. Invest Ophthalmol Vis Sci 2010; 51: 459–464.
Muller A, Hauk TG, Fischer D . Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain 2007; 130 (Pt 12): 3308–3320.
Fischer D . What are the principal mediators of optic nerve regeneration after inflammatory stimulation in the eye? Proc Natl Acad Sci USA 2010; 107: E8 author reply E9.
Hauk TG, Muller A, Lee J, Schwendener R, Fischer D . Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp Neurol 2008; 209: 469–482.
Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D . Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci 2009; 29: 14334–14341.
Fischer D . CNTF, a key factor mediating the beneficial effects of inflammatory reactions in the eye. Brain 2008; 131: e97.
Leibinger M, Muller A, Gobrecht P, Diekmann H, Andreadaki A, Fischer D . Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis 2013; 4: e609.
Muller A, Hauk TG, Leibinger M, Marienfeld R, Fischer D . Exogenous CNTF stimulates axon regeneration of retinal ganglion cells partially via endogenous CNTF. Mol Cell Neurosci 2009; 41: 233–246.
Lingor P, Tonges L, Pieper N, Bermel C, Barski E, Planchamp V et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain 2008; 131 (Pt 1): 250–263.
Park K, Luo JM, Hisheh S, Harvey AR, Cui Q . Cellular mechanisms associated with spontaneous and ciliary neurotrophic factor-cAMP-induced survival and axonal regeneration of adult retinal ganglion cells. J Neurosci 2004; 24: 10806–10815.
Leibinger M, Andreadaki A, Fischer D . Role of mTOR in neuroprotection and axon regeneration after inflammatory stimulation. Neurobiol Dis 2012; 46: 314–324.
Heskamp A, Leibinger M, Andreadaki A, Gobrecht P, Diekmann H, Fischer D . CXCL12/SDF-1 facilitates optic nerve regeneration. Neurobiol Dis 2013; 55: 76–86.
Cui Q, Benowitz L, Yin Y . Does CNTF mediate the effect of intraocular inflammation on optic nerve regeneration? Brain 2008; 131 (Pt 6): e96 author reply e97.
Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F et al. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat Neurosci 2006; 9: 843–852.
Rane SG, Reddy EP . Janus kinases: components of multiple signaling pathways. Oncogene 2000; 19: 5662–5679.
Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B et al. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 2008; 322: 963–966.
Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K et al. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron 2009; 64: 617–623.
Sun F, Park KK, Belin S, Wang D, Lu T, Chen G et al. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature 2011; 480: 372–375.
Grozdanov V, Muller A, Sengottuvel V, Leibinger M, Fischer D . A method for preparing primary retinal cell cultures for evaluating the neuroprotective and neuritogenic effect of factors on axotomized mature CNS neurons. Curr Protoc Neurosci 2010 Chapter 3: Unit3. 22.
Fischer D, Petkova V, Thanos S, Benowitz LI . Switching mature retinal ganglion cells to a robust growth state in vivo: gene expression and synergy with RhoA inactivation. J Neurosci 2004; 24: 8726–8740.
Bonilla IE, Tanabe K, Strittmatter SM . Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci 2002; 22: 1303–1315.
Peterson WM, Wang Q, Tzekova R, Wiegand SJ . Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci 2000; 20: 4081–4090.
Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C et al. Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci USA 2009; 106: 19587–19592.
Chamberlin NL, Du B, de Lacalle S, Saper CB . Recombinant adeno-associated virus vector: use for transgene expression and anterograde tract tracing in the CNS. Brain Res 1998; 793: 169–175.
Bartlett JS, Samulski RJ, McCown TJ . Selective and rapid uptake of adeno-associated virus type 2 in brain. Hum Gene Ther 1998; 9: 1181–1186.
Fischer D, He Z, Benowitz LI . Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neurosci 2004; 24: 1646–1651.
Sapieha PS, Peltier M, Rendahl KG, Manning WC, Di Polo A . Fibroblast growth factor-2 gene delivery stimulates axon growth by adult retinal ganglion cells after acute optic nerve injury. Mol Cell Neurosci 2003; 24: 656–672.
Harvey AR, Kamphuis W, Eggers R, Symons NA, Blits B, Niclou S et al. Intravitreal injection of adeno-associated viral vectors results in the transduction of different types of retinal neurons in neonatal and adult rats: a comparison with lentiviral vectors. Mol Cell Neurosci 2002; 21: 141–157.
Bareyre FM, Garzorz N, Lang C, Misgeld T, Buning H, Kerschensteiner M . In vivo imaging reveals a phase-specific role of STAT3 during central and peripheral nervous system axon regeneration. Proc Natl Acad Sci USA 2011; 108: 6282–6287.
Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE et al. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J 2012; 31: 1350–1363.
Qiu J, Cafferty WB, McMahon SB, Thompson SW . Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci 2005; 25: 1645–1653.
Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A . Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron 2012; 74: 1015–1022.
Smith RP, Lerch-Haner JK, Pardinas JR, Buchser WJ, Bixby JL, Lemmon VP . Transcriptional profiling of intrinsic PNS factors in the postnatal mouse. Mol Cell Neurosci 2011; 46: 32–44.
Miao T, Wu D, Zhang Y, Bo X, Subang MC, Wang P et al. Suppressor of cytokine signaling-3 suppresses the ability of activated signal transducer and activator of transcription-3 to stimulate neurite growth in rat primary sensory neurons. J Neurosci 2006; 26: 9512–9519.
Hellstrom M, Muhling J, Ehlert EM, Verhaagen J, Pollett MA, Hu Y et al. Negative impact of rAAV2 mediated expression of SOCS3 on the regeneration of adult retinal ganglion cell axons. Mol Cell Neurosci 2011; 46: 507–515.
Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K et al. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol 2002; 156: 287–297.
Alonzi T, Middleton G, Wyatt S, Buchman V, Betz UA, Muller W et al. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol Cell Neurosci 2001; 18: 270–282.
Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y . Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol 2001; 170: 63–71.
Nakazawa T, Tamai M, Mori N . Brain-derived neurotrophic factor prevents axotomized retinal ganglion cell death through MAPK and PI3K signaling pathways. Invest Ophthalmol Vis Sci 2002; 43: 3319–3326.
Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S . Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol 1998; 161: 4652–4660.
Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K et al. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 1999; 6: 973–985.
Acknowledgements
We thank Professor M. Sendtner, University of Würzburg, Germany, and Professor S. Akira, Osaka University, Japan for providing STAT3-floxed mice for this study and Professor Z. He, Children’s Hospital, Boston, USA, as well as Profesor K. Park, University of Miami, USA for providing the AAV2-Cre plasmid. We thank Marcel Kohlhaas, University of Düsseldorf for technical support. This work was supported by the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Edited by A Verkhratsky
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Leibinger, M., Andreadaki, A., Diekmann, H. et al. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis 4, e805 (2013). https://doi.org/10.1038/cddis.2013.310
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cddis.2013.310
Keywords
This article is cited by
-
Modification of the height of a weight drop traumatic brain injury model that causes the formation of glial scar and cognitive impairment in rats
BMC Neurology (2023)
-
Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice
Nature Communications (2021)
-
Signal transducer and activator of transcription-3 mediated neuroprotective effect of interleukin-6 on cobalt chloride mimetic hypoxic cell death in R28 cells
Molecular Biology Reports (2021)
-
Nogo-A-targeting antibody promotes visual recovery and inhibits neuroinflammation after retinal injury
Cell Death & Disease (2020)
-
Twist1 Plays an Anti-apoptotic Role in Mutant Huntingtin Expression Striatal Progenitor Cells
Molecular Neurobiology (2020)