Abstract
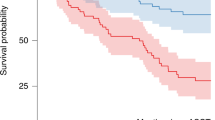
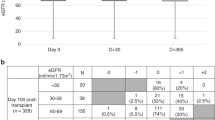
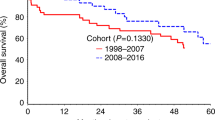
Autologous hematopoietic cell transplantation (AHCT) in multiple myeloma (MM) patients with renal insufficiency (RI) is controversial. Patients who underwent AHCT for MM between 2008 and 2013 were identified (N=1492) and grouped as normal/mild (⩾60 mL/min), N=1240, moderate (30–59), N=185 and severe RI (<30), N=67 based on Modification of Diet in Renal Disease. Multivariate analyses of non-relapse mortality (NRM), relapse, PFS and overall survival (OS) were performed. Of the 67 patients with severe RI, 35 were on dialysis prior to AHCT. Patients received melphalan 200 mg/m2 (Mel 200) in 92% (normal/mild), 75% (moderate) and 33% (severe) RI; remainder received 140 mg/m2 (Mel 140). Thirty four of 35 patients with severe RI achieved post-AHCT dialysis independence. The 5-year PFS for normal, moderate and severe RI was 35 (95% CI, 31–38)%, 40 (31–49)% and 27 (15–40)%, respectively, (P=0.42); 5-year OS for normal, moderate and severe RI was 68 (65–71)%, 68 (60–76)% and 60 (46–74)%, respectively, (P=0.69). With moderate RI, 5-year PFS for high-dose melphalan 140 mg/m2 was 18 (6–35)% and for Mel 200 was 46 (36–57)% (P=0.009). With severe RI, 5-year PFS Mel 140 was 25 (11–41) % and for Mel 200 was 32 (11–58)% (P=0.37). We conclude that AHCT is safe and effective in patients with MM with RI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Dimopoulos MA, Sonneveld P, Leung N, Merlini G, Ludwig H, Kastritis E et al. International myeloma working group recommendations for the diagnosis and management of myeloma-related renal impairment. J Clin Oncol 2016; 34: 1544–1557.
Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med 1996; 335: 91–97.
Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med 2003; 348: 1875–1883.
Cook G, Ashcroft AJ, Cairns DA, Williams CD, Brown JM, Cavenagh JD et al. The effect of salvage autologous stem-cell transplantation on overall survival in patients with relapsed multiple myeloma (final results from BSBMT/UKMF Myeloma X Relapse [Intensive]): a randomised, open-label, phase 3 trial. Lancet Haematol 2016; 3: e340–e351.
Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Eleutherakis-Papaiakovou E, Migkou M et al. Bortezomib-based triplets are associated with a high probability of dialysis independence and rapid renal recovery in newly diagnosed myeloma patients with severe renal failure or those requiring dialysis. Am J Hematol 2016; 91: 499–502.
Dimopoulos MA, Roussou M, Gkotzamanidou M, Nikitas N, Psimenou E, Mparmparoussi D et al. The role of novel agents on the reversibility of renal impairment in newly diagnosed symptomatic patients with multiple myeloma. Leukemia 2013; 27: 423–429.
Badros A, Barlogie B, Siegel E, Roberts J, Langmaid C, Zangari M et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol 2001; 114: 822–829.
El Fakih R, Fox P, Popat U, Nieto Y, Shah N, Parmar S et al. Autologous hematopoietic stem cell transplantation in dialysis-dependent myeloma patients. Clin Lymphoma Myeloma Leuk 2015; 15: 472–476.
Knudsen LM, Nielsen B, Gimsing P, Geisler C . Autologous stem cell transplantation in multiple myeloma: outcome in patients with renal failure. Eur J Haematol 2005; 75: 27–33.
Lee CK, Zangari M, Barlogie B, Fassas A, van Rhee F, Thertulien R et al. Dialysis-dependent renal failure in patients with myeloma can be reversed by high-dose myeloablative therapy and autotransplant. Bone Marrow Transplant 2004; 33: 823–828.
Raab MS, Breitkreutz I, Hundemer M, Benner A, Klaus J, Hegenbart U et al. The outcome of autologous stem cell transplantation in patients with plasma cell disorders and dialysis-dependent renal failure. Haematologica 2006; 91: 1555–1558.
St Bernard R, Chodirker L, Masih-Khan E, Jiang H, Franke N, Kukreti V et al. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplant 2015; 50: 95–99.
Weinstein R, Kershaw G, Bailey J, Greene M, Chhibber V, Vauthrin M et al. Safety and efficacy of autologous hemopoietic progenitor cell collection in tandem with hemodialysis in multiple myeloma with myeloma cast nephropathy. J Clin Apher 2014; 29: 83–89.
Sweiss K, Patel S, Culos K, Oh A, Rondelli D, Patel P . Melphalan 200 mg/m2 in patients with renal impairment is associated with increased short-term toxicity but improved response and longer treatment-free survival. Bone Marrow Transplant 2016; 51: 1337–1341.
Dimopoulos MA, Sonnevel P, Leung N, Merlini G, Ludwig H, Kastritis E et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J Clin Oncol 2016; 34: 1544–1557.
Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I et al. High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol 2016; 82: 149–159.
Shaw PJ, Nath CE, Lazarus HM . Not too little, not too much-just right! (Better ways to give high dose melphalan). Bone Marrow Transplant 2014; 49: 1457–1465.
Attal M, Lauwers-Cances V, Marit G, Caillot D, Moreau P, Facon T et al. Lenalidomide maintenance after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1782–1791.
McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG et al. Lenalidomide after stem-cell transplantation for multiple myeloma. N Engl J Med 2012; 366: 1770–1781.
Acknowledgements
This publication is funded in part by the Research and Education Program Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and by KL2TR001438 from the Clinical and Translational Science Award program of the National Center for Advancing Translational Sciences (D’Souza, A). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi USA; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the US Government *Corporate Members.
Author contributions
Conception and design: Anuj Mahindra, Parameswaran Hari, Raphael Fraser, Anita D’Souza. Collection and assembly of data: Anuj Mahindra, Parameswaran Hari, Raphael Fraser, Mingwei Fei, Anita D’Souza. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval: All authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
This work was presented in part as an oral presentation at the 59th Annual Meeting of the American Society of Hematology, San Diego, December 2016.
Rights and permissions
About this article
Cite this article
Mahindra, A., Hari, P., Fraser, R. et al. Autologous hematopoietic cell transplantation for multiple myeloma patients with renal insufficiency: a center for international blood and marrow transplant research analysis. Bone Marrow Transplant 52, 1616–1622 (2017). https://doi.org/10.1038/bmt.2017.198
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2017.198
This article is cited by
-
Autologous stem cell transplantation in multiple myeloma patients with renal impairment
Annals of Hematology (2023)
-
Safety and efficacy of autologous stem cell transplantation in dialysis-dependent myeloma patients—The DIADEM study from the chronic malignancies working party of the EBMT
Bone Marrow Transplantation (2023)
-
Autologous stem cell transplantation for multiple myeloma patients with chronic kidney disease: a safe and effective option
Bone Marrow Transplantation (2022)
-
Current status of autologous stem cell transplantation for multiple myeloma
Blood Cancer Journal (2019)
-
Utilization of hematopoietic stem cell transplantation for the treatment of multiple myeloma: a Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus statement
Bone Marrow Transplantation (2019)