Abstract
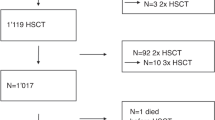
Gemtuzumab ozogamicin (GO) may increase the risk of sinusoidal obstruction syndrome (SOS) when used prior to allogeneic stem cell transplantation (HSCT). We assessed SOS incidence and outcomes after HSCT of 146 adults, with a median age of 50 years, previously receiving GO. SOS prophylaxis was used in 69 patients (heparin n=57, ursodeoxycholic acid n=8, defibrotide n=4). Cumulative incidence (CI) of SOS was 8% (n=11), with death in 3 patients. Median interval between last GO dose and HSCT was 130 days. Overall survival (OS) and SOS incidence did not differ for patients receiving GO ⩽3.5 months before HSCT and the others. CI of acute and chronic GVHD was 31% and 25%, respectively. Probability of OS and leukemia-free survival (LFS) at 5 years was 40% and 37%, respectively. Relapse incidence and non-relapse mortality were 42% and 21%, respectively. In multivariate analysis, active disease at HSCT was associated with relapse and worse LFS and OS (P<0.03). Liver abnormalities before HSCT correlated with worse OS (P<0.03). Use of low-dose GO prior to HSCT is associated with an acceptable SOS incidence. Prospective studies investigating the role and the utility of SOS prophylaxis are warranted.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen A et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant 2010; 16: 157–168.
Toh HC, McAfee SL, Sackstein R, Cox BF, Colby C, Spitzer TR . Late onset veno-occlusive disease following high-dose chemotherapy and stem cell transplantation. Bone Marrow Transplant 1999; 24: 891–895.
Hamann PR, Hinman LM, Hollander I, Beyer CF, Lindh D, Holcomb R et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody-calicheamicin conjugate for the treatment of acute myeloid leukemia. Bioconjug Chem 2002; 13: 47–58.
Larson RA, Sievers EL, Stadtmauer EA, Lowenberg B, Estey EH, Dombret H et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 2005; 104: 1442–1452.
Wadleigh M, Richardson PG, Zahrieh D, Lee S, Cutler C, Ho V et al. Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 2003; 102: 1578–1582.
Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 2013; 121: 4854–4860.
Burnett AK, Hills RK, Milligan D, Kieldsen L, Kell J, Russell NH et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol 2011; 29: 369–377.
Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol 2012; 30: 3924–3931.
Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 2012; 379: 1508–1516.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH et al. Refinement of cytogenetic classification in acute myeloid leukaemia: determination of prognostic significance of rarer recurring chromosomal abnormalities amongst 5,876 younger adult patients treated in the UK Medical Research Council trials. Blood 2010; 116: 354–365.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A longterm clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
National Cancer Institute. Common Terminology Criteria for Adverse Events v4.0. NCI, NIH, DHHS: 29 May 2009.
Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, Contentin N et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the ALFA group. Leukemia 2007; 21: 66–71.
Lee JL, Gooley T, Bensinger W, Schiffman K, McDonald GB . Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 1999; 5: 306–315.
McKoy JM, Angelotta C, Bennett CL, Tallman MS, Wadleigh M, Evens AM et al. Gemtuzumab ozogamicin-associated sinusoidal obstructive syndrome (SOS): an overview from the research on adverse drug events and reports (RADAR) project. Leuk Res 2007; 31: 599–604.
Kumar S, DeLeve LD, Kamath PS, Tefferi A . Hepatic veno-occlusive disease (sinusoidal obstruction syndrome) after hematopoietic stem cell transplantation. Mayo Clin Proc 2003; 78: 589–598.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Fine JP, Gray R . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Carreras E, Díaz-Beyá M, Rosiñol L, Martínez C, Fernández-Avilés F, Rovira M . The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant 2011; 17: 1713–1720.
Nagler A, Labopin M, Berger R, Bunjes D, Campos A, Socié G et al. Allogeneic hematopoietic SCT for adults AML using i.v. BU in the conditioning regimen: outcomes and risk factors for the occurrence of hepatic sinusoidal obstructive syndrome. Bone Marrow Transplant 2014; 49: 628–633.
Mohty M, Bay J, Faucher C, Choufi B, Bilger K, Tournilhac O et al. Graft-versus-host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin–based reduced-intensity preparative regimen. Blood 2003; 102: 470–476.
Chevallier P, Prebet T, Turlure P, Hunault M, Vigoroux S, Harousseau JL et al. Prior treatment with gemtuzumab ozogamicin and the risk of veno-occlusive disease after allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant 2010; 45: 165–170.
Attal BM, Huguet F, Rubie H, Huynh A, Charlet JP, Payen JL et al. Prevention of hepatic veno-occlusive disease after bone marrow transplantation by continuous infusion of low-dose heparin: a prospective, randomized trial. Blood 1992; 79: 2834–2840.
Marsa-Vila N, Gorin NC, Laporte JP, Labopin M, Dupuy-Montbrun MC, Fouillard L et al. Prophylactic heparin does not prevent liver veno-occlusive disease following autologous bone marrow transplantation. Eur J Hematol 1991; 47: 346–354.
Dignan F, Gujral D, Ethell M, Evans S, Treleaven J, Morgan G et al. Prophylactic defibrotide in allogeneic stem cell transplantation: minimal morbidity and zero mortality from vno-occlusive disease. Bone Marrow Transplant 2007; 40: 79–82.
Chalandon Y, Roosnek E, Mermillod B, Newton A, Ozsahin H, Wacker P et al. Prevention of veno-occlusive disease with defibrotide after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2004; 10: 347–354.
Acknowledgements
We thank Audrey Mailhol and Emmanuelle Polge for helping with data collection.
Author contributions
GB and MM designed the study. GB collected the data from the database of the ALWP of the EBMT (chaired by MM and AN). AC, RF, JEC, DB, MM, AR, NC, JMR, MS, JS, PAvdB, AB and GS were the principal investigators of the 11 highest recruiting centers of the study. ML, GB, MM and AN analyzed and interpreted the data. GB, MM and AN wrote or revised the manuscript, and all authors reviewed its final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
List of participating centers
List of participating centers
The following transplant centers (in alphabetical order of the country) contributed cases for the study: Department of Clinical Hematology, Universitair Ziekenhuis Brussel, Brussels, Belgium; Hématologie CHU Amiens, France, Institut Paoli Calmettes, Marseille, France; Hopital Saint Louis, Paris, France; Hopital Claude Huriez, Lille, France; Hopital A Michallon, Pole Cancérologie, Grenoble, France; Centre Hospitalier Lyon Sud, Pierre Benite, France; Hopital Saint Antoine, Paris, France; Centre Becquerel, Rouen, France; Hopital Saint Louis, Paris, France; Hannover Medical University, Hannover, Germany; Azienda Ospedaliero Universitaria S Maria della Misericordia di Udine, Italy; Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; ICO-Hospital Universitari Germans Trias i Pujol, Barcelona, Spain; Hospital Santa Creu i Sant Pau, Barcelona, Spain; Hospital Reina Sofia, Cordoba, Spain; The Christie Clinic, Manchester, United Kingdom.
Rights and permissions
About this article
Cite this article
Battipaglia, G., Labopin, M., Candoni, A. et al. Risk of sinusoidal obstruction syndrome in allogeneic stem cell transplantation after prior gemtuzumab ozogamicin treatment: a retrospective study from the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant 52, 592–599 (2017). https://doi.org/10.1038/bmt.2016.302
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.302
This article is cited by
-
Outcomes following hematopoietic stem cell transplantation in patients treated with standard chemotherapy with or without gemtuzumab ozogamicin for acute myeloid leukemia
Bone Marrow Transplantation (2021)
-
Effect of Sirolimus levels between days 11 and 20 after allogeneic stem cell transplantation on the risk of hepatic sinusoidal obstruction syndrome
Bone Marrow Transplantation (2021)
-
3+7 Combined Chemotherapy for Acute Myeloid Leukemia: Is It Time to Say Goodbye?
Current Oncology Reports (2021)
-
Liver disease during and after hematopoietic stem cell transplantation in adults: a single-center Egyptian experience
Journal of the Egyptian National Cancer Institute (2020)
-
Veno-occlusive disease/sinusoidal obstruction syndrome in patients with prior gemtuzumab ozogamicin: literature analysis of survival after defibrotide treatment
Blood Cancer Journal (2020)