Abstract
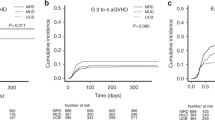
Few studies have presented a comparison of myeloablative cord blood transplantation (CBT) and HLA-identical sibling hematopoietic cell transplantation (HCT) for AML in a disease-specific analysis, and the evaluation of GvHD-free and relapse-free survival (GRFS) in AML patients after unrelated CBT has not been reported. A total of 162 consecutive AML patients receiving intensified myeloablative unrelated CBT (n=107) or allogeneic PBSC transplantation (allo-PBSCT) or bone marrow transplantation (BMT) from an HLA-identical sibling donor (n=55) were investigated. Neutrophil or platelet engraftment was slower in the CBT cohort compared with that in the allo-PBSCT/BMT cohort. The incidence of grade II–IV or grade III–IV acute GvHD (aGvHD) and transplant-related mortality (TRM) were not significantly different in the two cohorts. Compared with the allo-PBSCT/BMT cohort, the CBT cohort had a significantly lower rate of chronic GvHD (cGvHD) (13.7% vs 28.3%; P=0.047) or extensive cGvHD (9.9% vs 24.1%; hazard ratio (HR)=2.06, P=0.039). The incidence of relapse at 5 years in the CBT cohort was significantly lower than that in the allo-PBSCT/BMT cohort (15.3% vs 36.1%; HR=4.62, P=0.009). The probabilities of overall survival and leukemia-free survival were similar between the two cohorts. The adjusted 5-year probability of GRFS was higher after CBT than that after allo-PBSCT/BMT (55.4% vs 39.2%; HR=1.63, P=0.042). The present study suggests that, for AML patients, intensified myeloablative unrelated CBT is associated with less cGvHD and a lower risk of relapse. In addition, these patients do not experience excessive TRM or severe aGvHD that translates into better GRFS compared with those patients who undergo HLA-identical sibling allo-PBSCT/BMT; this observation may reflect the clinical separation between cGvHD and GvL within our CBT protocol.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rio B, Chevret S, Vigouroux S, Chevallier P, Fürst S, Sirvent A et al. Decreased nonrelapse mortality after unrelated cord blood transplantation for acute myeloid leukemia using reduced-intensity conditioning: a prospective phase II multicenter trial. Biol Blood Marrow Transplant 2015; 21: 445–453.
Devillier R, Harbi S, Fürst S, Crocchiolo R, El-Cheikh J, Castagna L et al. Poor outcome with nonmyeloablative conditioning regimen before cord blood transplantation for patients with high-risk acute myeloid leukemia compared with matched related or unrelated donor transplantation. Biol Blood Marrow Transplant 2014; 20: 1560–1565.
Zheng C, Zhu X, Tang B, Yao W, Song K, Tong J et al. Comparative analysis of unrelated cord blood transplantation and HLA-matched sibling hematopoietic stem cell transplantation in children with high-risk or advanced acute leukemia. Ann Hematol 2015; 94: 473–480.
Liu HL, Sun ZM, Geng LQ, Wang XB, Ding KY, Tong J et al. Similar survival, but better quality of life after myeloablative transplantation using unrelated cord blood vs matched sibling donors in adults with hematologic malignancies. Bone Marrow Transplant 2014; 49: 1063–1069.
Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG et al. Composite end point of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood 2015; 125: 1333–1338.
Zheng C, Zhu X, Tang B, Zhang L, Geng L, Liu H et al. The impact of pre-transplant minimal residual disease on outcome of intensified myeloablative cord blood transplant for acute myeloid leukemia in first or second complete remission. Leuk Lymphoma 2016; 57: 1398–1405.
Iacobelli S EBMT Statistical Committee. Suggestions on the use of statistical methodologies in studies of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant 2013; 48: S1–S37.
Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P et al. Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of acute graft-versus-host disease. Br J Haematol 2012; 158: 30–45.
Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P et al. Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. Diagnosis and management of chronic graft-versus-host disease. Br J Haematol 2012; 158: 46–61.
Konuma T, Kato S, Oiwa-Monna M, Ishii H, Tojo A, Takahashi S . Comparison of graft-versus-host disease-free, relapse-free survival of transplantation using matched sibling donor, matched unrelated donor or unrelated cord blood after myeloablative conditioning for adult patients with hematological malignancies. Leuk Lymphoma, (e-pub ahead of print 14 January 2016; doi:10.3109/10428194.2015.1122784).
Konuma T, Tsukada N, Kanda J, Uchida N, Ohno Y, Miyakoshi S et al. Donor/Source Working Group of the Japan Society for Hematopoietic Cell Transplantation. Comparison of transplant outcomes from matched sibling bone marrow or peripheral blood stem cell and unrelated cord blood in patients 50 years or older. Am J Hematol 2016; 91: E284–E292.
Kuwatsuka Y, Atsuta Y, Horowitz MM, Inagaki J, Kanda J, Kato K et al. Center for International Blood and Marrow Transplant Research (CIBMTR), and Donor/Source and GVHD Working Group of the Japan Society for Hematopoietic Cell Transplantation (JSHCT). Graft-versus-host disease and survival after cord blood transplantation for acute leukemia: a comparison of Japanese versus White populations. Biol Blood Marrow Transplant 2014; 20: 662–667.
Fiuza-Luces C, Simpson RJ, Ramírez M, Lucia A, Berger NA . Physical function and quality of life in patients with chronic GvHD: a summary of preclinical and clinical studies and a call for exercise intervention trials in patients. Bone Marrow Transplant 2016; 51: 13–26.
Jalali A, Alimoghaddam K, Mahmoudi M, Mohammad K, Mousavi SA, Bahar B et al. The effect of GVHD on long-term outcomes after peripheral blood allogeneic stem cell transplantation from an HLA-identical sibling in adult acute lymphocytic leukemia: a landmark analysis approach in competing risks. Int J Hematol Oncol Stem Cell Res 2014; 8: 1–8.
Arora M, Cutler CS, Jagasia MH, Pidala J, Chai X, Martin PJ et al. Late acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2016; 22: 449–455.
Lazaryan A, Weisdorf DJ, DeFor T, Brunstein CG, MacMillan ML, Bejanyan N et al. Risk factors for acute and chronic graft-versus-host disease after allogeneic hematopoietic cell transplantation with umbilical cord blood and matched sibling donors. Biol Blood Marrow Transplant 2016; 22: 134–140.
Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant 2013; 19: 904–911.
Chang YJ, Huang XJ . Haploidentical stem cell transplantation: anti-thymocyte globulin-based experience. Semin Hematol 2016; 53: 82–89.
Walker I, Panzarella T, Couban S, Couture F, Devins G, Elemary M et al. Canadian Blood and Marrow Transplant Group. Pretreatment with anti-thymocyte globulin versus no anti-thymocyte globulin in patients with haematological malignancies undergoing haemopoietic cell transplantation from unrelated donors: a randomised, controlled, open-label, phase 3, multicentre trial. Lancet Oncol 2016; 17: 164–173.
Zheng C, Luan Z, Fang J, Sun X, Chen J, Li CK et al. Comparison of conditioning regimens with or without antithymocyte globulin for unrelated cord blood transplantation in children with high-risk or advanced hematological malignancies. Biol Blood Marrow Transplant 2015; 21: 707–712.
Zheng C, Tang B, Yao W, Tong J, Zhu X, Song K et al. Comparison of unrelated cord blood transplantation and HLA-matched sibling hematopoietic stem cell transplantation for patients with chronic myeloid leukemia in advanced stage. Biol Blood Marrow Transplant 2013; 19: 1708–1712.
Yao W, Zheng CC, Liu HL, Geng LQ, Tang BL, Tong J et al. Salvaged single-unit cord blood transplantation for 26 patients with hematologic malignancies not in remission. Braz J Med Biol Res 2015; 48: 871–876.
Hiwarkar P, Qasim W, Ricciardelli I, Gilmour K, Quezada S, Saudemont A et al. Cord blood T cells mediate enhanced antitumor effects compared with adult peripheral blood T cells. Blood 2015; 126: 2882–2891.
Gupta T, Kannan S, Dantkale V, Laskar S . Cyclophosphamide plus total body irradiation compared with busulfan plus cyclophosphamide as a conditioning regimen prior to hematopoietic stem cell transplantation in patients with leukemia: a systematic review and meta-analysis. Hematol Oncol Stem Cell Ther 2011; 4: 17–29.
Mori T, Aisa Y, Watanabe R, Yamazaki R, Kato J, Shimizu T et al. Long-term follow-up of allogeneic hematopoietic stem cell transplantation for de novo acute myelogenous leukemia with a conditioning regimen of total body irradiation and granulocyte colony-stimulating factor-combined high-dose cytarabine. Biol Blood Marrow Transplant 2008; 14: 651–657.
Inamoto Y, Nishida T, Suzuki R, Miyamura K, Sao H, Iida H et al. Significance of additional high-dose cytarabine in combination with cyclophosphamide plus total body irradiation regimen for allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39: 25–30.
Arai Y, Takeda J, Aoki K, Kondo T, Takahashi S, Onishi Y et al. AML and MDS Working Group of Japan Society for Hematopoietic Cell Transplantation. Efficiency of high-dose cytarabine added to CY/TBI in cord blood transplantation for myeloid malignancy. Blood 2015; 126: 415–422.
Arai Y, Aoki K, Takeda J, Kondo T, Eto T, Ota S et al. AML and MDS Working Group of Japan Society for Hematopoietic Cell Transplantation. Clinical significance of high-dose cytarabine added to cyclophosphamide/total-body irradiation in bone marrow or peripheral blood stem cell transplantation for myeloid malignancy. J Hematol Oncol 2015; 8: 1–7.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 81570159 and 81470350), Anhui Provincial Natural Science Foundation of China (No. 1608085MH181) and Anhui Provincial Public Welfare Technology Application Research linkage project in 2015 (No. 1501ld04020).
Author contributions
C-CZ and Z-MS designed the study, performed the research, provided and analyzed the data. C-CZ wrote the paper. B-LT, X-YZ, X-HZ, LZ, L-QG and H-LL provided data, edited the paper and contributed to the analysis of research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Zheng, CC., Zhu, XY., Tang, BL. et al. Clinical separation of cGvHD and GvL and better GvHD-free/relapse-free survival (GRFS) after unrelated cord blood transplantation for AML. Bone Marrow Transplant 52, 88–94 (2017). https://doi.org/10.1038/bmt.2016.182
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.182
This article is cited by
-
Allogeneic hematopoietic cell transplantation with cord blood versus mismatched unrelated donor with post-transplant cyclophosphamide in acute myeloid leukemia
Journal of Hematology & Oncology (2021)
-
Low-dose antithymocyte globulin inhibits chronic graft-versus-host disease in peripheral blood stem cell transplantation from unrelated donors
Bone Marrow Transplantation (2021)
-
Low-dose anti-thymocyte globulin for GVHD prophylaxis in HLA-matched allogeneic peripheral blood stem cell transplantation
Bone Marrow Transplantation (2021)