Abstract
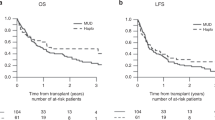
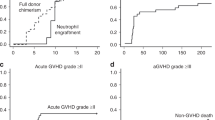
A comparison was conducted of 213 patients with haematologic malignancies who underwent HLA-identical sibling (n=108) or HLA-haploidentical (n=105) haematopoietic cell transplantation (haplo-HCT) at our centre. The conditioning regimen included fludarabine, busulphan, cyclophosphamide and antilymphocyte globulin (ATG) (FBCA). The total dose of ATG differed between identical and haploidentical groups (3.75 mg/kg versus 12.5 mg/kg). The cumulative incidences of grade II–IV acute GvHD in the identical and haploidentical groups were 20.4% and 21.9% (P=0.73), and 2-year cumulative incidences of chronic GvHD were 36.4% and 24.1% (P=0.17), respectively. The 3-year probabilities of non-relapse mortality for identical and haploidentical groups were 20.5% and 34.9% (P=0.048), and for relapse were 22.2% and 21.0% (P=0.85), respectively. The 3-year overall survivals in the identical and haploidentical groups were 62.6% and 52.6% (P=0.054), whereas the 3-year disease-free survivals were 54.7% and 43.1% (P=0.14), respectively. In the multivariate analysis, patients in the high-risk group exhibited reduced survival, and the higher dose of mononuclear or CD34+ cells resulted in an increase in the likelihood of survival. In conclusion, haplo-HCT based on an FBCA conditioning regimen could achieve nearly comparable outcomes to HLA-identical sibling HCT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Reisner Y, Kapoor N, Kirkpatrick D, Pollack MS, Dupont B, Good RA et al. Transplantation for acute leukaemia with HLA-A and B nonidentical parental marrow cells fractionated with soybean agglutinin and sheep red blood cells. Lancet 1981; 2: 327–331.
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 2006; 38: 291–297.
Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F et al. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant 2013; 19: 117–122.
Lu DP, Dong LJ, Wu T, Huang XJ, Zhang MJ, Han W et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 2006; 107: 3065–3073.
Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood 2011; 118: 282–288.
Giralt S, Logan B, Rizzo D, Zhang M-J, Ballen K, Emmanouilides C et al. Reduced-intensity conditioning for unrelated donor progenitor cell transplantation: long-term follow-up of the first 285 reported to the National Marrow Donor Program. Biol Blood Marrow Transplant 2007; 13: 844–852.
Lin X, Lu ZG, Song CY, Huang YX, Guo KY, Deng L et al. Long-term outcome of HLA-haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion based on an FBCA conditioning regimen for hematologic malignancies. Bone Marrow Transplant 2015; 50: 1092–1097.
Schlenk RF, Dohner K, Mack S, Stoppel M, Kiraly F, Gotze K et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol 2010; 28: 4642–4648.
O'Donnell MR, Tallman MS, Abboud CN, Altman JK, Appelbaum FR, Arber DA et al. Acute myeloid leukemia, version 2.2013. J Natl Compr Canc Netw 2013; 11: 1047–1055.
Burnett A, Wetzler M, Lowenberg B . Therapeutic advances in acute myeloid leukemia. J Clin Oncol 2011; 29: 487–494.
Bachanova V, Weisdorf D . Unrelated donor allogeneic transplantation for adult acute lymphoblastic leukemia: a review. Bone Marrow Transplant 2008; 41: 455–464.
Sullivan KM, Agura E, Anasetti C, Appelbaum F, Badger C, Bearman S et al. Chronic graft-versus-host disease and other late complications of bone marrow transplantation. Semin Hematol 1991; 28: 250–259.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995 15: 825–828.
Gooley TA, Leisenring W, Crowley J, Storer BE . Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med 1999; 18: 695–706.
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015; 125: 3956–3962.
Wang Y, Liu DH, Liu KY, Xu LP, Zhang XH, Han W et al. Long-term follow-up of haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for the treatment of leukemia: nine years of experience at a single center. Cancer 2013; 119: 978–985.
Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood 2014; 124: 2735–2743.
Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 2011; 17: 821–830.
Finke J, Schmoor C, Lang H, Potthoff K, Bertz H . Matched and mismatched allogeneic stem-cell transplantation from unrelated donors using combined graft-versus-host disease prophylaxis including rabbit anti-T lymphocyte globulin. J Clin Oncol 2003; 21: 506–513.
Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant 2006; 12: 560–565.
Kumar A, Mhaskar AR, Reljic T, Mhaskar RS, Kharfan-Dabaja MA, Anasetti C et al. Antithymocyte globulin for acute-graft-versus-host-disease prophylaxis in patients undergoing allogeneic hematopoietic cell transplantation: a systematic review. Leukemia 2012; 26: 582–588.
Remberger M, Mattsson J, Ringden O . Polyclonal anti-T-cell globulin as part of the preparative regimen for pediatric allogeneic stem-cell transplantation. Pediatr Transplant 2001; 5: 285–292.
Acknowledgements
We acknowledge the great contributions of the nurses, physicians and laboratory staff from the Department of Hematology, Zhujiang Hospital, Southern Medical University, China, for their patient care. This work was supported by the Scientific and Technology Programs of Guangzhou, China (No.: 2011Y1-00033-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Long, H., Lu, Z., Song, C. et al. Long-term outcomes of HLA-haploidentical stem cell transplantation based on an FBCA conditioning regimen compared with those of HLA-identical sibling stem cell transplantation for haematologic malignancies. Bone Marrow Transplant 51, 1470–1475 (2016). https://doi.org/10.1038/bmt.2016.170
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2016.170
This article is cited by
-
Potent immunomodulation and angiogenic effects of mesenchymal stem cells versus cardiomyocytes derived from pluripotent stem cells for treatment of heart failure
Stem Cell Research & Therapy (2019)
-
Assessing the efficacy of an ambulatory peripheral blood hematopoietic stem cell transplant program using reduced intensity conditioning in a low-middle-income country
Bone Marrow Transplantation (2019)
-
Everyone has a donor: contribution of the Chinese experience to global practice of haploidentical hematopoietic stem cell transplantation
Frontiers of Medicine (2019)
-
The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology
Journal of Hematology & Oncology (2018)
-
A review of infectious complications after haploidentical hematopoietic stem cell transplantations
Infection (2017)