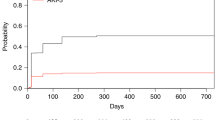
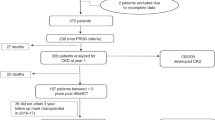
Abstract
Acute kidney injury (AKI) is highly prevalent whether the patients undergo myeloablative or non-myeloablative hematopoietic cell transplantation (HCT); however, the pathogenesis and risk factors leading to AKI can differ between the two. The prognosis of AKI in patients receiving HCT is poor. In fact, AKI following HCT is associated not only with increased short- and long-term mortality, but also with progression to chronic kidney disease. Herein, the authors provide a comprehensive and up-to-date review of the definition and diagnosis, as well as of the incidence, pathogenesis and outcome of AKI in patients undergoing HCT, centering on the differences between myeloablative and non-myeloablative regimens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Schattenberg AV, Levenga TH . Differences between the different conditioning regimens for allogeneic stem cell transplantation. Curr Opin Oncol 2006; 18: 667–670.
Dey BR, Spitzer TR . Current status of haploidentical stem cell transplantation. Br J Haematol 2006; 135: 423–437.
Niederwieser D, Maris M, Shizuru JA, Petersdorf E, Hegenbart U, Sandmaier BM et al. Low-dose total body irradiation (TBI) and fludarabine followed by haematopoietic cell transplantation (HCT) from HLA-matched or mismatched unrelated donors and postgrafting immunosuppression with cyclosporine and mycophenolate mofetil (MMF) can induce durable complete chimerism and sustained remissions in patients with haematologic diseases. Blood 2003; 101: 1620–1629.
Blaise D, Bay JO, Faucher C, Michallet M, Boiron J-M, Choufi B et al. Reduced-intensity preparative regimen and allogeneic stem cell transplantation for advanced solid tumors. Blood 2004; 103: 435–441.
Belkacemi Y, Labopin M, Hennequin C, Hoffstetter S, Mungai R, Wygoda M et al. Reduced-intensity conditioning regimen using low-dose total body irradiation before allogeneic transplant for hematologic malignancies: experience from the European Group for Blood and Marrow Transplantation. Int J Radiat Oncol Biol Phys 2007; 67: 544–551.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P . Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204–R212.
Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31.
Lopes JA, Jorge S . The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clin Kidney J 2013; 6: 8–14.
Lopes JA, Jorge S, Silva S, de Almeida E, Abreu F, Martins C et al. Acute renal failure following myeloablative autologous and allogeneic haematopoietic cell transplantation. Bone Marrow Transplant 2006; 38: 707.
Lopes JA, Jorge S, Silva S, de Almeida E, Abreu F, Martins C et al. Prognostic utility of the acute kidney injury network (AKIN) criteria for acute kidney injury in myeloablative haematopoietic cell transplantation. Bone Marrow Transplant 2007; 40: 1005–1006.
Ando M, Mori J, Ohashi K, Akiyama H, Morito T, Tsuchiya K et al. A comparative assessment of the RIFLE, AKIN and conventional criteria for acute kidney injury after hematopoietic SCT. Bone Marrow Transplant 2010; 45: 1427–1434.
Lopes JA, Gonçalves S, Jorge S, Raimundo M, Resende L, Lourenço F et al. Contemporary analysis of the influence of the acute kidney injury after reduced intensity conditioning haematopoietic cell transplantation on long-term survival. Bone Marrow Transplant 2008; 42: 619–626.
Liu H, Li Y-F, Liu B-C, Ding J-H, Chen B-A, Xu W-L et al. A multicenter, retrospective study of acute kidney injury in adult patients with nonmyeloablative hematopoietic SCT. Bone Marrow Transplant 2010; 45: 153–158.
KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl (2011) 2012; 2: 1–138 Available at http://www.kdigo.org/clinical_practice_guidelines/AKI.php.
Canet E, Lengline E, Zafrani L, Peraldi M-N, Socié G, Azoulay E . Acute kidney injury in critically ill allo-HSCT recipients. Bone Marrow Transplant 2014; 49: 1121–1122.
Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet 2005; 365: 1231–1238.
Singer E, Elger A, Elitok S, Kettritz R, Nickolas TL, Barasch J et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int 2011; 80: 405–414.
Ricci Z, Luciano R, Favia I, Garisto C, Muraca M, Morelli S et al. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit Care 2011; 15: R160.
Kumpers P, Hafer C, Lukasz A, Lichtinghagen R, Brand K, Fliser D et al. Serum neutrophil gelatinase-associated lipocalin at inception of renal replacement therapy predicts survival in critically ill patients with acute kidney injury. Crit Care 2010; 14: R9.
Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G et al. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol 2011; 57: 1752–1761.
Taghizadeh-Ghehi M, Sarayani A, Ashouri A, Ataei S, Moslehi A, Hadjibabaie M . Urine neutrophil gelatinase associated lipocalin as an early marker of acute kidney injury in hematopoietic stem cell transplantation patients. Ren Fail 2015; 37: 994–998.
Shingai N, Morito T, Najima Y, Igarashi A, Kobayashi T, Doki N, Kakihana K et al. Urinary liver-type fatty acid-binding protein linked with increased risk of acute kidney injury after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20: 2010–2014.
Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B . Nephrotic syndrome after hematopoietic cell transplantation: do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol 2006; 1: 685–694.
Kusumi E, Kami M, Hara S, Hoshino J, Yamaguchi Y, Murashige N et al. Postmortem examination of the kidney in allogeneic hematopoietic stem cell transplantation recipients: possible involvement of graft-versus-host disease. Int J Hematol 2008; 87: 225–230.
Kersting S, Dorp SV, Theobald M, Verdonck LF . Acute renal failure after nonmyeloablative stem cell transplantation in adults. Biol Blood Marrow Transplant 2008; 14: 125–131.
Kersting S, Koomans HA, Hené RJ, Verdonck LF . Acute renal failure after allogeneic myeloablative stem cell transplantation: retrospective analysis of incidence, risk factors and survival. Bone Marrow Transplant 2007; 39: 359–365.
Irazabal MV, Eirin A, Gertz MA, Dispenzieri A, Kumar S, Buadi FK et al. Acute kidney injury during leukocyte engraftment after autologous stem cell transplantation in patients with light-chain amyloidosis. Am J Hematol 2012; 87: 51–54.
Spitzer TR . Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 2001; 27: 893–898.
Shingai N, Morito T, Najima Y, Kobayashi T, Doki N, Kakihana K et al. Early-onset acute kidney injury is a poor prognostic sign for allogeneic SCT recipients. Bone Marrow Transplant 2015; 50: 1557–1562.
Cohen EP . Renal failure after bone marrow transplantation. Lancet 2001; 357: 6–7.
Noel C, Hazzan M, Noel-Walter MP, Jouet JP . Renal failure and bone marrow transplantation. Nephrol Dial Transplant 1998; 13: 2464–2466.
Pinãna JL, Valcárcel D, Martino R, Barba P, Moreno E, Sureda et al. Study of kidney function impairment after reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. A single-center experience. Biol Blood Marrow Transplant 2009; 15: 21–29.
Parikh CR, Sandmaier BM, Storb RF, Blume KG, Sahebi F, Maloney DG et al. Acute renal failure after nonmyeloablative hematopoietic cell transplantation. J Am Soc Nephrol 2004; 15: 1868–1876.
Naesens M, Kuypers DR, Sarwal M . Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol 2009; 4: 481–508.
Lamas S . Cellular mechanisms of vascular injury mediated by calcineurin inhibitors. Kidney Int 2005; 68: 898–907.
Perazella MA . Crystal-induced acute renal failure. Am J Med 1999; 106: 459–465.
Barbanti-Brodano G, Martini F, De Matei M, Lazzarin L, Corallini A, Tognon M . BK and JC human polyomaviruses and simian virus 40: natural history of infection in humans, experimental oncogenicity, and association with human tumors. Adv Virus Res 1998; 50: 69–99.
Mylonakis D, Goes N, Rubin RH, Cosimi AB, Colvin RB, Fishman JA . BK virus in solid organ transplant recipients: an emerging syndrome. Transplantation 2001; 72: 1587–1592.
Dropulic LK, Jones RJ . Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant 2008; 41: 11–18.
Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC . BK virus and the Impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant 2010; 10: 407–415.
Leung AY, Mak R, Lie AK, Yuen KY, Cheng VC, Liang R et al. Clinicopathological feature and risk factors of clinically overt hemorrhagic cystitis complicating bone marrow transplantation. Bone Marrow Transplant 2002; 29: 509–513.
Erard V, Kim HW, Corey L, Limaye A, Huang ML, Myerson D et al. BK DNAviral load in plasma: evidence for an association with hemorrhagic cystitis in allogeneic hematopoietic cell transplant recipients. Blood 2005; 106: 1130–1132.
Baldwin A, Kingman H, Darville M, Foot AB, Grier D, Cornish JM et al. Outcome and clinical course of 100 patients with adenovirus infection following bone marrow transplantation. Bone Marrow Transplant 2000; 26: 1333–1338.
Ito M, Hirabayashi N, Uno Y, Nakayama A, Asai J . Necrotizing tubulointerstitial nephritis associated with adenovirus infection. Hum Pathol 1991; 22: 1225–1231.
Bruno B, Zager RA, Boeckh MJ, Gooley TA, Myerson DH, Huang ML . Adenovirus nephritis in hematopoietic stem-cell transplantation. Transplantation 2004; 77: 1049–1057.
Bil-Lula I, Ussowicz M, Rybka B, Wendycz-Domalewska D, Ryczan R, Gorczyńska E et al. Hematuria due to adenoviral infection in bone marrow transplant recipients. Transplant Proc 2010; 42: 3729–3734.
Hatakeyama N, Suzuki N, Kudoh T, Hori T, Mizue N, Tsutsumi H . Successful cidofovir treatment of adenovirus-associated hemorrhagic cystitis and renal dysfunction after allogeneic bone marrow transplantation. Pediatr Infect Dis J 2003; 22: 928–929.
Fanourgiakis P, Georgala A, Vekemans M, Triffet A, De Bruyn JM, Duchateau V . Intravesical instillation of cidofovir in the treatment of hemorrhagic cystitis caused by adenovirus type 11 in a bone marrow transplant recipient. Clin Infect Dis 2005; 40: 199–201.
Zager RA . Acute renal failure in the setting of bone marrow transplantation. Kidney Int 1994; 46: 1443–1458.
Lam AQ, Humphreys BD . Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol 2012; 7: 1692–1700.
Coppell JA, Richardson PG, Soiffer R, Martin PL, Kernan NA, Chen et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant 2010; 16: 157–168.
McDonald GB, Hinds MS, Fisher LD . Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
Carreras E, Bertz H, Arcese W, Vernant JP, Tomás JF, Hagglund H et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. Blood 1998; 92: 3599–3604.
Parikh CR, Coca SG . Acute renal failure in hematopoietic cell transplantation. Kidney Int 2006; 69: 430–435.
Dulley FL, Kanfer EJ, Appelbaum FR, Amos D, Hill RS, Buckner CD et al. Venoocclusive disease of the liver after chemoradiotherapy and autologous bone marrow transplantation. Transplantation 1987; 43: 870–873.
DeLeve LD, Shulman HM, McDonald GB . Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002; 22: 27–42.
Litzow MR, Repoussis PD, Schroeder G, Schembri-Wismayer D, Batts KP, Anderson PM et al. Veno-occlusive disease of the liver after blood and marrow transplantation: analysis of pre- and post-transplant risk factors associated with severity and results of therapy with tissue plasminogen activator. Leuk Lymphoma 2002; 43: 2099–2107.
Richardson PG, Soiffer RJ, Antin JH, Uno H, Jin Z, Kurtzberg J et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol Blood Marrow Transplant 2010; 16: 1005–1017.
Reddy P . Pathophysiology of acute graft-versus-host disease. Hematol Oncol 2003; 21: 149–161.
Sadeghi B, Al-Chaqmaqchi H, Al-Hashmi S, Brodin D, Hassan Z, Abedi-Valugerdi M et al. Early-phase GVHD gene expression profile in target versus non-target tissues: kidney, a possible target? Bone Marrow Transplant 2013; 48: 284–293.
Hahn T, Rondeau C, Shaukat A, Jupudy V, Miller A, Alam AR et al. Acute renal failure requiring dialysis after allogeneic blood and marrow transplantation identifies very poor prognosis patients. Bone Marrow Transplant 2003; 32: 405–410.
Higo S, Shimizu A, Masuda Y, Nagasaka S, Kajimoto Y et al. Acute graft-versus-host disease of the kidney in allogeneic rat bone marrow transplantation. PLoS ONE 2014; 9: e115399.
Hingorani S, Finn LS, Pao E, Lawler R, Schoch G, McDonald GB et al. Urinary elafin and kidney injury in hematopoietic cell transplant recipients. Clin J Am Soc Nephrol 2015; 10: 12–20.
Cho BS, Yahng SA, Lee SE, Eom KS, Kim YJ, Kim HJ et al. Validation of recently proposed consensus criteria for thrombotic microangiopathy after allogeneic hematopoietic stem-cell transplantation. Transplantation 2010; 90: 918–926.
Keir L, Coward RJ . Advances in our understanding of the pathogenesis of glomerular thrombotic microangiopathy. Pediatr Nephrol 2011; 26: 523–533.
Changsirikulchai S, Myerson D, Guthrie KA, McDonald GB, Alpers CE, Hingorani SR . Renal thrombotic microangiopathy after hematopoietic cell transplant: role of GVHD in pathogenesis. Clin J Am Soc Nephrol 2009; 4: 345–353.
Siami K, Kojouri K, Swisher KK, Selby GB, George JN, Laszik ZG . Thrombotic microangiopathy after allogeneic hematopoietic stem cell transplantation: an autopsy study. Transplantation 2008; 85: 22–28.
Chan GS, Lam MF, Au WY, Chim S, Tse KC, Lo SH et al. Clinicopathologic analysis of renal biopsies after haematopoietic stem cell transplantation. Nephrology (Carlton) 2008; 13: 322–330.
Laskin BL, Goebel J, Davies SM, Jodele S . Small vessels, big trouble in the kidneys and beyond: hematopoietic stem cell transplantation–associated thrombotic microangiopathy. Blood 2011; 118: 1452–1462.
Goldberg RJ, Nakagawa T, Johnson RJ, Thurman JM . The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis 2010; 56: 1168–1174.
Thachil J . Nitric oxide in transplantation-related thrombotic microangiopathy. Bone Marrow Transplant 2009; 43: 513–514.
George JN, Li X, McMinn JR, Terrell DR, Vesely SK, Selby GB . Thrombotic thrombocytopenic purpura-hemolytic uremic syndrome following allogeneic HPC transplantation: a diagnostic dilemma. Transfusion 2004; 44: 294–304.
Cruz DN, Perazella MA, Mahnensmith RL . Bone marrow transplant nephropathy: a case report and review of the literature. J Am Soc Nephrol 1997; 8: 166–173.
Batts ED, Lazarus HM . Diagnosis and treatment of transplantation-associated thrombotic microangiopathy: real progress or are we still waiting? Bone Marrow Transplant 2007; 40: 709–719.
George JN . Hematopoietic stem cell transplantation-associated thrombotic microangiopathy: defining a disorder. Bone Marrow Transplant 2008; 41: 917–918.
Choi CM, Schmaier AH, Snell MR, Lazarus HM . Thrombotic microangiopathy in haematopoietic stem cell transplantation: diagnosis and treatment. Drugs 2009; 69: 183–198.
Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS . De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis 2003; 41: 471–479.
Sakai M, Ikezoe T, Bandobashi K, Togitani K, Yokoyama A . Successful treatment of transplantation-associated thrombotic microangiopathy with recombinant human soluble thrombomodulin. Bone Marrow Transplant 2010; 45: 803–805.
Fadia A, Casserly LF, Sanchorawala V, Seldin DC, Wright DG, Skinner M et al. Incidence and outcome of acute renal failure complicating autologous stem cell transplantation for AL amyloidosis. Kidney Int 2003; 63: 1868–1873.
Hingorani SR, Guthrie K, Batchelder A, Schoch G, Aboulhosn N, Manchion J et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney Int 2005; 67: 272–277.
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106: 2912–2919.
Kagoya Y, Kataoka K, Yasuhito Nannya Y, Kurokawa M . Pretransplant predictors and posttransplant sequels of acute kidney injury after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17: 394–400.
Parikh CR, McSweeney PA, Korular D, Ecder T, Merouani A, Taylor J et al. Renal dysfunction in allogeneic hematopoietic cell transplantation. Kidney Int 2002; 62: 566–573.
Parikh CR, Schrier RW, Storer B, Diaconescu R, Sorror ML, Maris MB et al. Comparison of ARF after myeloablative and nonmyeloablative hematopoietic cell transplantation. Am J Kidney Dis 2005; 45: 502–509.
Ostermann M, Chang RW . Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35: 1837–1843.
Lopes JA, Fernandes P, Jorge S, Goncalves S, Alvarez A, Costa e Silva Z et al. Acute kidney injury in intensive care unit patients: a comparison between the RIFLE and the Acute Kidney Injury Network classifications. Crit Care 2008; 12: R110, 16-31.
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR . Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53: 961–973.
Weiss AS, Sandmaier BM, Storer B, Storb R, McSweeney PA, Parikh CR . Chronic kidney disease following non-myeloablative hematopoietic cell transplantation. Am J Transplant 2006; 6: 89–94.
Hingorani S, Guthrie KA, Schoch G, Weiss NS, McDonald GB . Chronic kidney disease in long-term survivors of hematopoietic cell transplant. Bone Marrow Transplant 2007; 39: 223–229.
Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE . Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant 2010; 16: 515–524.
Shimoi T, Ando M, Munakata W, Kobayashi T, Kakihana K, Ohashi K et al. The significant impact of acute kidney injury on CKD in patients who survived over 10 years after myeloablative allogeneic SCT. Bone Marrow Transplant 2013; 48: 80–84.
Ferenbach D, Bonventre J . Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 2015; 11: 264–276.
Basile DP . Rarefaction of peritubular capillaries following ischemic acute renal failure. A potential factor predisposing to progressive nephropathy. Curr Opin Nephrol Hypertens 2004; 13: 1–7.
Spurgeon-Pechman KR, Donohoe DL, Mattson DL, Lund H, James L, Basile DP . Recovery from acute renal failure predisposes hypertension and secondary renal disease in response to elevated sodium. Am J Physiol Renal Physiol 2007; 293: F269–F278.
Basile DP . The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int 2007; 72: 151–156.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305.
Sarafidis PA, Bakris GL . Microalbuminuria and chronic kidney disease as risk factors for cardiovascular disease. Nephrol Dial Transplant 2006; 21: 2366–2374.
Lewington S, Clarke R, Qizilbash N, Peto R, Collins R . Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360: 1903–1913.
Lai CF, Wu VC, Huang TM, Yeh YC, Wang KC, Han YY et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 2012; 16: R123.
Li X, Hassoun HT, Santora R, Rabb H . Organ crosstalk: the role of the kidney. Curr Opin Crit Care 2009; 15: 481–487.
Jang HR, Rabb H . The innate immune response in ischemic acute kidney injury. Clin Immunol 2009; 130: 41–50.
Vincent IS, Okusa MD . Biology of renal recovery: molecules, mechanisms, and pathways. Nephron Clin Pract 2014; 127: 10–14.
Martina MN, Noel S, Bandapalle S, Hamad AR, Rabb H . T lymphocytes and acute kidney injury: update. Nephron Clin Pract 2014; 127: 51–55.
Parikh CR, Yarlagadda SG, Storer B, Sorror M, Storb R, Sandmaier B . Impact of acute kidney injury on long-term mortality after nonmyeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant 2008; 14: 309–315.
Lopes JA, Jorge S, Gonçalves S, Resina C, Silva S, de Almeida E et al. Contemporary analysis of the influence of acute kidney injury (AKI) after myeloablative hematopoietic cell transplantation on long-term patient’s survival. Bone Marrow Transplant 2008; 42: 139–141.
Rabb H . The promise of immune cell therapy for acute kidney injury. J Clin Invest 2012; 122: 3852–3854.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Lopes, J., Jorge, S. & Neves, M. Acute kidney injury in HCT: an update. Bone Marrow Transplant 51, 755–762 (2016). https://doi.org/10.1038/bmt.2015.357
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.357
This article is cited by
-
Analysis of risk factors for fatal renal complications after allogeneic hematopoietic cell transplantation
Bone Marrow Transplantation (2024)
-
Incidence of acute kidney injury after hematopoietic stem cell transplantation in children: a systematic review and meta-analysis
European Journal of Pediatrics (2023)
-
Kidney complications in 107 Fanconi anemia patients submitted to hematopoietic cell transplantation
European Journal of Pediatrics (2022)
-
Acute kidney injury in cancer patients
Clinical and Experimental Nephrology (2022)
-
Acute kidney injury in pediatric hematopoietic cell transplantation: critical appraisal and consensus
Pediatric Nephrology (2022)