Abstract
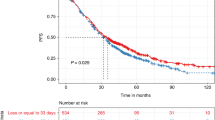
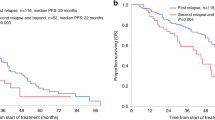
Little is known about the prognostic impact of prior paclitaxel therapy and response to induction chemotherapy defined as the regimen preceding high-dose chemotherapy (HDCT) for the salvage therapy of advanced germ cell tumors. Twenty European Society for Blood and Marrow Transplantation centers contributed data on patients treated between 2002 and 2012. Paclitaxel used in either prior lines of therapy or in induction-mobilization regimens was considered. Multivariable Cox analyses of prespecified factors were undertaken on PFS and overall survival (OS). As of October 2013, data for 324 patients had been contributed to this study. One hundred and ninety-two patients (59.3%) had received paclitaxel. Sixty-one patients (19%) had a progression to induction chemotherapy, 234 (72%) a response (29 (9%) missing or granulocyte colony-stimulating factor without chemotherapy). Both progression to induction chemotherapy and prior paclitaxel were significantly associated with shorter OS univariably (P<0.001 and P=0.032). On multivariable analysis from the model with fully available data (N=216) progression to induction was significantly prognostic for PFS and OS (P=0.003), but prior paclitaxel was not (P=0.674 and P=0.739). These results were confirmed after multiple imputation of missing data. Progression to induction chemotherapy could be demonstrated as an independent prognostic factor, in contrast to prior paclitaxel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R . High-dose chemotherapy and stem-cell rescue for metastatic germ-cell tumors. N Engl J Med 2007; 357: 340–348.
Lorch A, Bascoul-Mollevi C, Kramar A, Einhorn L, Necchi A, Massard C et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: evidence from a large international database. J Clin Oncol 2011; 29: 2178–2184.
Kondagunta GV, Bacik J, Sheinfeld J, Bajorin D, Bains M, Reich L et al. Paclitaxel plus ifosfamide followed by high-dose carboplatin plus etoposide in previously treated germ cell tumors. J Clin Oncol 2007; 25: 85–90.
Feldman DR, Sheinfeld J, Bajorin DF, Fischer P, Turkula S, Ishill N et al. TI-CE high-dose chemotherapy for patients with previously treated germ cell tumors: results and prognostic factor analysis. J Clin Oncol 2010; 28: 1706–1713.
Pico JL, Rosti G, Kramar A, Wandt H, Koza V, Salvioni R et al. A randomised trial of high-dose chemotherapy in the salvage treatment of patients failing first-line platinum chemotherapy for advanced germ cell tumours. Ann Oncol 2004; 15: 79–87.
International Prognostic Factors Study Group, Lorch A, Beyer J, Bascoul-Mollevi C, Kramar A, Einhorn LH et al. Prognostic factors in patients with metastatic germ cell tumors who experienced treatment failure with cisplatin-based first-line chemotherapy. J Clin Oncol 2010; 28: 4906–4911.
Beyer J, Kramar A, Mandanas R, Linkesch W, Greinix A, Droz JP et al. High-dose chemotherapy as salvage treatment in germ cell tumors: a multivariate analysis of prognostic variables. J Clin Oncol 1996; 14: 2638–2645.
Kondagunta GV, Bacik J, Donadio A, Bajorin D, Marion S, Sheinfeld J et al. Combination of paclitaxel, ifosfamide, and cisplatin is an effective second-line therapy for patients with relapsed testicular germ-cell tumors. J Clin Oncol 2005; 23: 6549–6555.
Nichols CR, Tricot G, Williams SD, van Besien K, Loehrer PJ, Roth BJ et al. Dose-intensive chemotherapy in refractory germ cell cancer: a phase I/II trial of high-dose carboplatin and etoposide with autologous bone marrow transplantation. J Clin Oncol 1989; 7: 932–939.
The International Germ Cell Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol 1997; 15: 594–603.
Peduzzi P, Concato J, Feinstein AR, Holford TR . Importance of number of events per independent variable in proportional hazards regression analysis, II: accuracy and precision of regression estimates. J Clin Epidemiol 1995; 48: 1503–1510.
Clark TG, Altman DG . Developing a prognostic model in the presence of missing data: an ovarian cancer case study. J Clin Epidemiol 2003; 56: 28–37.
Van Buuren S, Boshuizen HC, Knook DL . Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999; 18: 681–694.
Fizazi K, Pagliaro L, Laplanche A, Fléchon A, Mardiak J, Geoffrois L et al. Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG13): a phase 3, multicentre, randomised trial. Lancet Oncol 2014; 15: 1442–1450.
Necchi A, Lanza F, Rosti G, Martino M, Farè E, Pedrazzoli P . High-dose chemotherapy for germ cell tumors: do we have a model? Expert Opin Biol Ther 2015; 15: 33–44.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Presented in oral session, Annual Meeting of the European Society for Blood and Marrow Transplantation (EBMT), Istanbul, Turkey, 23−25 March 2015. Presented in poster session, Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, USA, 29 May−2 June 2015.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Necchi, A., Miceli, R., Bregni, M. et al. Prognostic impact of progression to induction chemotherapy and prior paclitaxel therapy in patients with germ cell tumors receiving salvage high-dose chemotherapy in the last 10 years: a study of the European Society for Blood and Marrow Transplantation Solid Tumors Working Party. Bone Marrow Transplant 51, 384–390 (2016). https://doi.org/10.1038/bmt.2015.300
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.300
This article is cited by
-
Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019
Bone Marrow Transplantation (2019)
-
Secondary malignancies after high-dose chemotherapy in germ cell tumor patients: a 34-year retrospective study of the European Society for Blood and Marrow Transplantation (EBMT)
Bone Marrow Transplantation (2018)