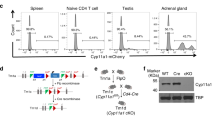
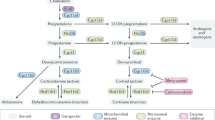
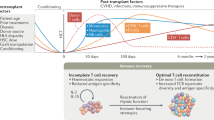
Abstract
Age-related decline in thymic function is a well-described process that results in reduced T-cell development and thymic output of new naïve T cells. Thymic involution leads to reduced response to vaccines and new pathogens in otherwise healthy individuals; however, reduced thymic function is particularly detrimental in clinical scenarios where the immune system is profoundly depleted such as after chemotherapy, radiotherapy, infection and shock. Poor thymic function and restoration of immune competence has been correlated with an increased risk of opportunistic infections, tumor relapse and autoimmunity. Apart from their primary role in sex dimorphism, sex steroid levels profoundly affect the immune system in general and, in fact, age-related thymic involution has been at least partially attributed to the increase in sex steroids at puberty. Subsequently it has been demonstrated that the removal of sex steroids, or sex steroid ablation (SSA), triggers physiologic changes that ultimately lead to thymic re-growth and improved T-cell reconstitution in settings of hematopoietic stem cell transplant (HSCT). Although the cellular and molecular process underlying these regenerative effects are still poorly understood, SSA clearly represents an attractive therapeutic approach to enhance thymic function and restore immune competence in immunodeficient individuals.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Shaw AC, Joshi S, Greenwood H, Panda A, Lord JM . Aging of the innate immune system. Curr Opin Immunol 2010; 22: 507–513.
Aw D, Silva AB, Palmer DB . Immunosenescence: emerging challenges for an ageing population. Immunology 2007; 120: 435–446.
Yung RL, Julius A . Epigenetics, aging, and autoimmunity. Autoimmunity 2008; 41: 329–335.
Miller RA . The aging immune system: primer and prospectus. Science 1996; 273: 70–74.
Burns EA, Leventhal EA . Aging, immunity, and cancer. Cancer Control 2000; 7: 513–522.
Castle SC . Clinical relevance of age-related immune dysfunction. Clin Infect Dis 2000; 31: 578–585.
Ershler WB, Longo DL . Aging and cancer: issues of basic and clinical science. J Natl Cancer Inst 1997; 89: 1489–1497.
Ciofani M, Zuniga-Pflucker JC . The thymus as an inductive site for T lymphopoiesis. Annu Rev Cell Dev Biol 2007; 23: 463–493.
Takahama Y . Journey through the thymus: stromal guides for T-cell development and selection. Nat Rev Immunol 2006; 6: 127–135.
Rothenberg EV, Moore JE, Yui MA . Launching the T-cell-lineage developmental programme. Nat Rev Immunol 2008; 8: 9–21.
Alves NL, Huntington ND, Rodewald HR, Di Santo JP . Thymic epithelial cells: the multi-tasking framework of the T cell ‘cradle’. Trends Immunol 2009; 30: 468–474.
Flores KG, Li J, Sempowski GD, Haynes BF, Hale LP . Analysis of the human thymic perivascular space during aging. J Clin Invest 1999; 104: 1031–1039.
Linton PJ, Dorshkind K . Age-related changes in lymphocyte development and function. Nat Immunol 2004; 5: 133–139.
Kurashima C, Utsuyama M, Kasai M, Ishijima SA, Konno A, Hirokawa K et al. The role of thymus in the aging of Th cell subpopulations and age-associated alteration of cytokine production by these cells. Int Immunol 1995; 7: 97–104.
Sempowski GD, Gooding ME, Liao HX, Le PT, Haynes BF . T cell receptor excision circle assessment of thymopoiesis in aging mice. Mol Immunol 2002; 38: 841–848.
Steinmann GG, Klaus B, Muller-Hermelink HK . The involution of the ageing human thymic epithelium is independent of puberty. A morphometric study. Scand J Immunol 1985; 22: 563–575.
George AJ, Ritter MA . Thymic involution with ageing: obsolescence or good housekeeping? Immunol Today 1996; 17: 267–272.
Gruver AL, Hudson LL, Sempowski GD . Immunosenescence of ageing. J Pathol 2007; 211: 144–156.
Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289: 179–186.
Fiore AE, Bridges CB, Cox NJ . Seasonal influenza vaccines. Curr Top Microbiol Immunol 2009; 333: 43–82.
McElhaney JE, Dutz JP . Better influenza vaccines for older people: what will it take? J Infect Dis 2008; 198: 632–634.
Nikolich-Zugich J . T cell aging: naive but not young. J Exp Med 2005; 201: 837–840.
Maraninchi D, Gluckman E, Blaise D, Guyotat D, Rio B, Pico JL et al. Impact of T-cell depletion on outcome of allogeneic bone-marrow transplantation for standard-risk leukaemias. Lancet 1987; 2: 175–178.
Storek J, Gooley T, Witherspoon RP, Sullivan KM, Storb R . Infectious morbidity in long-term survivors of allogeneic marrow transplantation is associated with low CD4 T cell counts. Am J Hematol 1997; 54: 131–138.
Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol 2001; 115: 630–641.
Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH et al. Comparison of immune reconstitution after unrelated and related T-cell- depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood 1999; 93: 467–480.
Storek J, Joseph A, Espino G, Dawson MA, Douek DC, Sullivan KM et al. Immunity of patients surviving 20 to 30 years after allogeneic or syngeneic bone marrow transplantation. Blood 2001; 98: 3505–3512.
Storek J, Witherspoon RP, Storb R . T cell reconstitution after bone marrow transplantation into adult patients does not resemble T cell development in early life. Bone Marrow Transplant 1995; 16: 413–425.
Small TN, Papadopoulos EB, Boulad F, Black P, Castro-Malaspina H, Childs BH et al. Comparison of immune reconstitution after unrelated and related T-cell-depleted bone marrow transplantation: effect of patient age and donor leukocyte infusions. Blood 1999; 93: 467–480.
Lehrnbecher T, Foster C, Vazquez N, Mackall CL, Chanock SJ . Therapy-induced alterations in host defense in children receiving therapy for cancer. J Pediatr Hematol Oncol 1997; 19: 399–417.
Wils EJ, van der Holt B, Broers AE, Posthumus-van Sluijs SJ, Gratama JW, Braakman E et al. Insufficient recovery of thymopoiesis predicts for opportunistic infections in allogeneic hematopoietic stem cell transplant recipients. Haematologica 2011; 96: 1846–1854.
Kim DH, Sohn SK, Won DI, Lee NY, Suh JS, Lee KB et al. Rapid helper T-cell recovery above 200 × 10 6/l at 3 months correlates to successful transplant outcomes after allogeneic stem cell transplantation. Bone Marrow Transplant 2006; 37: 1119–1128.
Sfikakis PP, Gourgoulis GM, Moulopoulos LA, Kouvatseas G, Theofilopoulos AN, Dimopoulos MA et al. Age-related thymic activity in adults following chemotherapy-induced lymphopenia. Eur J Clin Invest 2005; 35: 380–387.
Mackall CL, Fleisher TA, Brown MR, Andrich MP, Chen CC, Feuerstein IM et al. Age, thymopoiesis, and CD4+ T-lymphocyte regeneration after intensive chemotherapy. N Engl J Med 1995; 332: 143–149.
Dudakov JA, Hanash AM, Jenq RR, Young LF, Ghosh A, Singer NV et al. Interleukin-22 drives endogenous thymic regeneration in mice. Science 2012; 336: 91–95.
Dixit VD, Yang H, Sun Y, Weeraratna AT, Youm YH, Smith RG et al. Ghrelin promotes thymopoiesis during aging. J Clin Invest 2007; 117: 2778–2790.
Alpdogan O, Muriglan SJ, Kappel BJ, Doubrovina E, Schmaltz C, Schiro R et al. Insulin-like growth factor-I enhances lymphoid and myeloid reconstitution after allogeneic bone marrow transplantation. Transplantation 2003; 75: 1977–1983.
Li L, Hsu HC, Stockard CR, Yang P, Zhou J, Wu Q et al. IL-12 inhibits thymic involution by enhancing IL-7- and IL-2-induced thymocyte proliferation. J Immunol 2004; 172: 2909–2916.
Mackall CL, Fry TJ, Bare C, Morgan P, Galbraith A, Gress RE et al. IL-7 increases both thymic-dependent and thymic-independent T-cell regeneration after bone marrow transplantation. Blood 2001; 97: 1491–1497.
Fry TJ, Sinha M, Milliron M, Chu YW, Kapoor V, Gress RE et al. Flt3 ligand enhances thymic-dependent and thymic-independent immune reconstitution. Blood 2004; 104: 2794–2800.
Zakrzewski JL, Kochman AA, Lu SX, Terwey TH, Kim TD, Hubbard VM et al. Adoptive transfer of T-cell precursors enhances T-cell reconstitution after allogeneic hematopoietic stem cell transplantation. Nat Med 2006; 12: 1039–1047.
van den Brink MR, Alpdogan O, Boyd RL . Strategies to enhance T-cell reconstitution in immunocompromised patients. Nat Rev Immunol 2004; 4: 856–867.
Goldberg GL, Zakrzewski JL, Perales MA, van den Brink MR . Clinical strategies to enhance T cell reconstitution. Semin Immunol 2007; 19: 289–296.
Chen L, Xiao S, Manley NR . Foxn1 is required to maintain the postnatal thymic microenvironment in a dosage-sensitive manner. Blood 2009; 113: 567–574.
Hauri-Hohl MM, Zuklys S, Keller MP, Jeker LT, Barthlott T, Moon AM et al. TGF-beta signaling in thymic epithelial cells regulates thymic involution and postirradiation reconstitution. Blood 2008; 112: 626–634.
Andrew D, Aspinall R . Age-associated thymic atrophy is linked to a decline in IL-7 production. Exp Gerontol 2002; 37: 455–463.
Min H, Montecino-Rodriguez E, Dorshkind K . Reduction in the developmental potential of intrathymic T cell progenitors with age. J Immunol 2004; 173: 245–250.
Sempowski GD, Hale LP, Sundy JS, Massey JM, Koup RA, Douek DC et al. Leukemia inhibitory factor, oncostatin M, IL-6, and stem cell factor mRNA expression in human thymus increases with age and is associated with thymic atrophy. J Immunol 2000; 164: 2180–2187.
Sharp A, Kukulansky T, Globerson A . In vitro analysis of age-related changes in the developmental potential of bone marrow thymocyte progenitors. Eur J Immunol 1990; 20: 2541–2546.
Eren R, Zharhary D, Abel L, Globerson A . Age-related changes in the capacity of bone marrow cells to differentiate in thymic organ cultures. Cell Immunol 1988; 112: 449–455.
Gray DH, Seach N, Ueno T, Milton MK, Liston A, Lew AM et al. Developmental kinetics, turnover, and stimulatory capacity of thymic epithelial cells. Blood 2006; 108: 3777–3785.
Chen Y, Qiao S, Tuckermann J, Okret S, Jondal M . Thymus-derived glucocorticoids mediate androgen effects on thymocyte homeostasis. FASEB J 2010; 24: 5043–5051.
Windmill KF, Meade BJ, Lee VW . Effect of prepubertal gonadectomy and sex steroid treatment on the growth and lymphocyte populations of the rat thymus. Reprod Fertil Dev 1993; 5: 73–81.
Grossman CJ, Sholiton LJ, Blaha GC, Nathan P . Rat thymic estrogen receptor—II. Physiological properties. J Steroid Biochem 1979; 11: 1241–1246.
Kuhl H, Gross M, Schneider M, Weber W, Mehlis W, Stegmuller M et al. The effect of sex steroids and hormonal contraceptives upon thymus and spleen on intact female rats. Contraception 1983; 28: 587–601.
Luster MI, Hayes HT, Korach K, Tucker AN, Dean JH, Greenlee WF et al. Estrogen immunosuppression is regulated through estrogenic responses in the thymus. J Immunol 1984; 133: 110–116.
Dulos GJ, Bagchus WM . Androgens indirectly accelerate thymocyte apoptosis. Int Immunopharmacol 2001; 1: 321–328.
Olsen NJ, Olson G, Viselli SM, Gu X, Kovacs WJ . Androgen receptors in thymic epithelium modulate thymus size and thymocyte development. Endocrinology 2001; 142: 1278–1283.
McCruden AB, Stimson WH . Androgen binding cytosol receptors in the rat thymus: physicochemical properties, specificity and localisation. Thymus 1981; 3: 105–117.
Kovacs WJ, Olsen NJ . Androgen receptors in human thymocytes. J Immunol 1987; 139: 490–493.
Pearce P, Khalid BA, Funder JW . Androgens and the thymus. Endocrinology 1981; 109: 1073–1077.
Zoller AL, Kersh GJ . Estrogen induces thymic atrophy by eliminating early thymic progenitors and inhibiting proliferation of beta-selected thymocytes. J Immunol 2006; 176: 7371–7378.
Guevara Patino JA, Marino MW, Ivanov VN, Nikolich-Zugich J . Sex steroids induce apoptosis of CD8+CD4+ double-positive thymocytes via TNF-alpha. Eur J Immunol 2000; 30: 2586–2592.
Lai KP, Lai JJ, Chang P, Altuwaijri S, Hsu JW, Chuang KH et al. Targeting thymic epithelia AR enhances T-cell reconstitution and bone marrow transplant grafting efficacy. Mol Endocrinol 2013; 27: 25–37.
Henderson J . On the relationship of the thymus to the sexual organs: I. The influence of castration on the thymus. J Physiol 1904; 31: 222–229.
Goodall A . The post-natal changes in the thymus of guinea-pigs, and the effect of castration on thymus structure. J Physiol 1905; 32: 191–198.
Fitzpatrick FT, Kendall MD, Wheeler MJ, Adcock IM, Greenstein BD . Reappearance of thymus of ageing rats after orchidectomy. J Endocrinol 1985; 106: R17–R19.
Utsuyama M, Hirokawa K . Hypertrophy of the thymus and restoration of immune functions in mice and rats by gonadectomy. Mech Ageing Dev 1989; 47: 175–185.
Olsen NJ, Watson MB, Henderson GS, Kovacs WJ . Androgen deprivation induces phenotypic and functional changes in the thymus of adult male mice. Endocrinology 1991; 129: 2471–2476.
Oner H, Ozan E . Effects of gonadal hormones on thymus gland after bilateral ovariectomy and orchidectomy in rats. Arch Androl 2002; 48: 115–126.
Heng TS, Goldberg GL, Gray DH, Sutherland JS, Chidgey AP, Boyd RL et al. Effects of castration on thymocyte development in two different models of thymic involution. J Immunol 2005; 175: 2982–2993.
Heng TS, Reiseger JJ, Fletcher AL, Leggatt GR, White OJ, Vlahos K et al. Impact of sex steroid ablation on viral, tumour and vaccine responses in aged mice. PLoS ONE 2012; 7: e42677.
Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H et al. Augmentation of T Cell Levels and Responses Induced by Androgen Deprivation. J Immunol 2004; 173: 6098–6108.
Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno T, Vlahos K et al. Sex steroid ablation enhances immune reconstitution following cytotoxic antineoplastic therapy in young mice. J Immunol 2010; 184: 6014–6024.
Sutherland JS, Goldberg GL, Hammett MV, Uldrich AP, Berzins SP, Heng TS et al. Activation of thymic regeneration in mice and humans following androgen blockade. J Immunol 2005; 175: 2741–2753.
Goldberg GL, Alpdogan O, Muriglan SJ, Hammett MV, Milton MK, Eng JM et al. Enhanced immune reconstitution by sex steroid ablation following allogeneic hemopoietic stem cell transplantation. J Immunol 2007; 178: 7473–7484.
Goldberg GL, Sutherland JS, Hammet MV, Milton MK, Heng TS, Chidgey AP et al. Sex steroid ablation enhances lymphoid recovery following autologous hematopoietic stem cell transplantation. Transplantation 2005; 80: 1604–1613.
Dudakov JA, Goldberg GL, Reiseger JJ, Vlahos K, Chidgey AP, Boyd RL et al. Sex steroid ablation enhances hematopoietic recovery following cytotoxic antineoplastic therapy in aged mice. J Immunol 2009; 183: 7084–7094.
Goldberg GL, King CG, Nejat RA, Suh DY, Smith OM, Bretz JC et al. Luteinizing hormone-releasing hormone enhances T cell recovery following allogeneic bone marrow transplantation. J Immunol 2009; 182: 5846–5854.
Perisic M, Stojic-Vukanic Z, Pilipovic I, Kosec D, Nacka-Aleksic M, Dikic J et al. Role of ovarian hormones in T-cell homeostasis: from the thymus to the periphery. Immunobiology 2013; 218: 353–367.
Min H, Montecino-Rodriguez E, Dorshkind K . Reassessing the role of growth hormone and sex steroids in thymic involution. Clin Immunol 2006; 118: 117–123.
Umathe SN, Dixit PV, Wanjari MM, Ullewar MP . Leuprolide—a GnRH agonist—prevents restraint stress-induced immunosuppression via sex steroid-independent peripheral mechanism in mice. Int Immunopharmacol 2008; 8: 71–79.
Sutherland JS, Spyroglou L, Muirhead JL, Heng TS, Prieto-Hinojosa A, Prince HM et al. Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade. Clin Cancer Res 2008; 14: 1138–1149.
van Poppel H, Nilsson S . Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 2008; 71: 1001–1006.
Acknowledgements
This research was supported by the National Institutes of Health award nos R01-HL069929 (MRMvdB), R01-AI080455 (MRMvdB), R01-AI101406 (MRMvdB), P01-CA023766 (RJO) and Project 4 of P01-CA023766 (MRMvdB). Support was also received from the US National Institute of Allergy and Infectious Diseases (NIAID Contract HHSN272200900059C), the Experimental Therapeutics Center of MSKCC funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, the Lymphoma Foundation, Alex's Lemonade Stand, the Geoffrey Beene Cancer Research Center at MSKCC and the Susan and Peter Solomon Divisional Genomics Program. This project has received funding from the European Union’s Seventh Programme for Research, Technological Development and demonstration under grant agreement no (602587). EV was supported by fellowships from the Italian Foundation for Cancer Research and the Italian Society of Pharmacology and a New Investigator Award from the American Society for Blood and Marrow Transplantation. JAD was supported by a CJ Martin fellowship from the Australian National Health and Medical Research Council; a Scholar Award from the American Society of Hematology; and a K99 career transition award from the National Institutes of Health (1K99CA176376).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor MRMvdB has received consulting or advisory fees from Merck & Co, Yobira Therapeutics, Boehringer Ingelheim, and has received lecture fees from Merck & Co, Novartis Pharma A.G., Novartis Institute for Biomedical Research, Tobira Therapeutics, Boehringer Ingelheim and Regeneron Pharmaceuticals. He has also received grant support from Radiation Effects Research Foundation (RERF), NIH/NHLBI, NIH/NCI, NIH/NIAID and the European Consortium. The remaining authors declare no conflicts of interest.
Additional information
This article was published as part of a supplement, supported by WIS-CSP Foundation, in collaboration with Gilead, Milteny Biotec, Gamida cell, Adienne Pharma and Biotech, Medac hematology, Kiadis Pharma, Almog Diagnostic.
Rights and permissions
About this article
Cite this article
Velardi, E., Dudakov, J. & van den Brink, M. Sex steroid ablation: an immunoregenerative strategy for immunocompromised patients. Bone Marrow Transplant 50 (Suppl 2), S77–S81 (2015). https://doi.org/10.1038/bmt.2015.101
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2015.101
This article is cited by
-
Epigenetic modifications in thymic epithelial cells: an evolutionary perspective for thymus atrophy
Clinical Epigenetics (2021)
-
Curcumin protects thymus against D-galactose-induced senescence in mice
Naunyn-Schmiedeberg's Archives of Pharmacology (2021)
-
The role of the thymus in allogeneic bone marrow transplantation and the recovery of the peripheral T-cell compartment
Seminars in Immunopathology (2021)
-
Interventions to restore appropriate immune function in the elderly
Immunity & Ageing (2018)
-
Progress on thymic function from Maui
Nature Immunology (2016)