Abstract
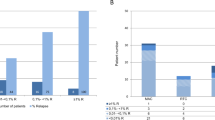
Detection of increasing mixed chimerism (IMC) using standard PCR correlates with relapse after allo-SCT for acute leukemias (ALs). Quantitative real-time PCR of insertion/deletion polymorphism (indel qrtPCR) is a much more sensitive method, which can be performed on peripheral blood. We studied the significance of low increases of recipient cells (0.1%) detected by indel qrtPCR in a cohort of 89 transplants. We did not observe relapse among the 32 patients with no IMC. Fifty-seven patients presented a first IMC, which was followed by four different scenarios: a decreasing MC (26 cases, no relapse), a stable MC (1 case, 1 relapse), a second IMC (24 cases, 15 relapse) or no control of chimerism (6 cases, 5 relapses). In multivariate analysis, detection of two successive IMCs was strongly associated with relapse (hazard ratio (HR): 9.4, 95% confidence interval (CI): 3.8–23; P<0.0001). Among the 57 patients who presented at least one IMC, 27 underwent immunomodulation (tapering of immunosuppression or donor lymphocyte injection), leading to a 1-year relapse rate of 15.7% vs 57.6% in the 30 other patients (P=0.0007). Altogether, these results indicate that chimerism analysis using indel qrtPCR in peripheral blood is a useful tool for detection of relapse in patients transplanted for AL.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Frassoni F, Barrett AJ, Grañena A, Ernst P, Garthon G, Kolb HJ et al. Relapse after a llogeneic bone marrow transplantation for acute leukaemia: a survey by the E.B.M.T. of 117 cases. Br J Haematol 1988; 70: 317–320.
Kumar L . Leukemia: management of relapse after allogeneic bone marrow transplantation. J Clin Oncol 1994; 12: 1710–1717.
Mortimer J, Blinder MA, Schulman S, Appelbaum FR, Buckner CD, Clift RA et al. Relapse of acute leukemia after marrow transplantation: natural history and results of subsequent therapy. J Clin Oncol 1989; 7: 50–57.
Bishop MR, Dean RM, Steinberg SM, Odom J, Pavletic SZ, Chow C et al. Clinical evidence of a graft-versus-lymphoma effect against relapsed diffuse large B-cell lymphoma after allogeneic hematopoietic stem-cell transplantation. Ann Oncol 2008; 19: 1935–1940.
Bloor AJC, Thomson K, Chowdhry N, Verfuerth S, Ings SJ, Chakraverty R et al. High response rate to donor lymphocyte infusion after allogeneic stem cell transplantation for indolent non-Hodgkin lymphoma. Biol Blood Marrow Transplant 2008; 14: 50–58.
Loren AW, Porter DL . Donor leukocyte infusions for the treatment of relapsed acute leukemia after allogeneic stem cell transplantation. Bone Marrow Transplant 2008; 41: 483–493.
Mehta J, Powles R, Treleaven J, Horton C, Meller S, Pinkerton CR et al. Outcome of acute leukemia relapsing after bone marrow transplantation: utility of second transplants and adoptive immunotherapy. Bone Marrow Transplant 1997; 19: 709–719.
Porter DL, Antin JH . The graft-versus-leukemia effects of allogeneic cell therapy. Annu Rev Med 1999; 50: 369–386.
Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R et al. Prospective trial of chemotherapy and donor leukocyte infusions for relapse of advanced myeloid malignancies after allogeneic stem-cell transplantation. J Clin Oncol 2002; 20: 405–412.
Choi S-J, Lee J-H, Lee J-H, Kim S, Seol M, Lee Y-S et al. Treatment of relapsed acute myeloid leukemia after allogeneic bone marrow transplantation with chemotherapy followed by G-CSF-primed donor leukocyte infusion: a high incidence of isolated extramedullary relapse. Leukemia 2004; 18: 1789–1797.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–2050.
Collins RH Jr, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Porter DL, Collins RH Jr, Hardy C, Kernan NA, Drobyski WR, Giralt S et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood 2000; 95: 1214–1221.
Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A et al. Donor lymphocyte infusion in the treatment of first hematological relapse after allogeneic stem-cell transplantation in adults with acute myeloid leukemia: a retrospective risk factors analysis and comparison with other strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol 2007; 25: 4938–4945.
Yan C-H, Liu D-H, Liu K-Y, Xu L-P, Liu Y-R, Chen H et al. Risk stratification-directed donor lymphocyte infusion could reduce relapse of standard-risk acute leukemia patients after allogeneic hematopoietic stem cell transplantation. Blood 2012; 119: 3256–3262.
Deol A, Lum LG . Role of donor lymphocyte infusions in relapsed hematological malignancies after stem cell transplantation revisited. Cancer Treat Rev 2010; 36: 528–538.
Barrios M, Jiménez-Velasco A, Román-Gómez J, Madrigal ME, Castillejo JA, Torres A et al. Chimerism status is a useful predictor of relapse after allogeneic stem cell transplantation for acute leukemia. Haematologica 2003; 88: 801–810.
Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 2009; 94: 1613–1617.
Scheffold C, Kroeger M, Zuehlsdorf M, Tchinda J, Silling G, Bisping G et al. Prediction of relapse of acute myeloid leukemia in allogeneic transplant recipients by marrow CD34+ donor cell chimerism analysis. Leukemia 2004; 18: 2048–2050.
Dominietto A . Minimal residual disease markers before and after allogeneic hematopoietic stem cell transplantation in acute myeloid leukemia. Curr Opin Hematol 2011; 18: 381–387.
Bacher U, Haferlach T, Fehse B, Schnittger S, Kröger N . Minimal residual disease diagnostics and chimerism in the post-transplant period in acute myeloid leukemia. ScientificWorldJournal 2011; 11: 310–319.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002; 99: 4618–4625.
Kletzel M, Huang W, Olszewski M, Khan S . Validation of chimerism in pediatric recipients of allogeneic hematopoietic stem cell transplantation (HSCT) a comparison between two methods: real-time PCR (qPCR) vs. variable number tandem repeats PCR (VNTR PCR). Chimerism 2013; 4: 1–8.
Horky O, Mayer J, Kablaskova L, Razga F, Krejci M, Kissova J et al. Increasing hematopoietic microchimerism is a reliable indicator of incipient AML relapse. Int J Lab Hematol 2011; 33: 57–66.
Jiménez-Velasco A, Barrios M, Román-Gómez J, Navarro G, Buño I, Castillejo JA et al. Reliable quantification of hematopoietic chimerism after allogeneic transplantation for acute leukemia using amplification by real-time PCR of null alleles and insertion/deletion polymorphisms. Leukemia 2005; 19: 336–343.
Wiedemann B, Klyuchnikov E, Kröger N, Zabelina T, Stahl T, Zeschke S et al. Chimerism studies with quantitative real-time PCR in stem cell recipients with acute myeloid leukemia. Exp Hematol 2010; 38: 1261–1271.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Lion T, Watzinger F, Preuner S, Kreyenberg H, Tilanus M, de Weger R et al. The EuroChimerism concept for a standardized approach to chimerism analysis after allogeneic stem cell transplantation. Leukemia 2012; 26: 1821–1828.
Bader P, Beck J, Frey A, Schlegel PG, Hebarth H, Handgretinger R et al. Serial and quantitative analysis of mixed hematopoietic chimerism by PCR in patients with acute leukemias allows the prediction of relapse after allogeneic BMT. Bone Marrow Transplant 1998; 21: 487–495.
Mattsson J, Uzunel M, Tammik L, Aschan J, Ringdén O . Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia 2001; 15: 1976–1985.
Koldehoff M, Steckel NK, Hlinka M, Beelen DW, Elmaagacli AH . Quantitative analysis of chimerism after allogeneic stem cell transplantation by real-time polymerase chain reaction with single nucleotide polymorphisms, standard tandem repeats, and Y-chromosome-specific sequences. Am J Hematol 2006; 81: 735–746.
Borchers S, Weissinger EM, Pabst B, Ganzenmueller T, Dammann E, Luther S et al. Expansion of recipient-derived antiviral T cells may influence donor chimerism after allogeneic stem cell transplantation. Transpl Infect Dis 2013; 15: 627–633.
Kröger N, Miyamura K, Bishop MR . Minimal residual disease following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2011; 17: S94–100.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011; 118: 5681–5688.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Handgretinger R, Lang P et al. Increasing mixed chimerism is an important prognostic factor for unfavorable outcome in children with acute lymphoblastic leukemia after allogeneic stem-cell transplantation: possible role for pre-emptive immunotherapy? J Clin Oncol 2004; 22: 1696–1705.
Bader P, Kreyenberg H, Hoelle W, Dueckers G, Kremens B, Dilloo D et al. Increasing mixed chimerism defines a high-risk group of childhood acute myelogenous leukemia patients after allogeneic stem cell transplantation where pre-emptive immunotherapy may be effective. Bone Marrow Transplant 2004; 33: 815–821.
Zeiser R, Spyridonidis A, Wäsch R, Ihorst G, Grüllich C, Bertz H et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia 2005; 19: 814–821.
Acknowledgements
We thank Dr Martine Torres for her editorial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Author contributions
NJ collected and analyzed the data and wrote the paper; SN, AG, MU, VL and JPV provided patients and samples and contributed in writing the manuscript; JLG performed statistical analysis; DB provided chimerism analysis, analyzed the data and contributed in writing the manuscript; ND provided patients and samples, analyzed the data and contributed in writing the manuscript.
Rights and permissions
About this article
Cite this article
Jacque, N., Nguyen, S., Golmard, JL. et al. Chimerism analysis in peripheral blood using indel quantitative real-time PCR is a useful tool to predict post-transplant relapse in acute leukemia. Bone Marrow Transplant 50, 259–265 (2015). https://doi.org/10.1038/bmt.2014.254
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.254
This article is cited by
-
Sequential high-sensitivity mutational and chimerism analyses predict responses to post-transplant salvage therapies in MDS
Bone Marrow Transplantation (2023)
-
Can novel methods replace the gold standard chimerism method after allogeneic hematopoietic stem cell transplantation?
Annals of Hematology (2023)
-
Chimerism analysis for clinicians: a review of the literature and worldwide practices
Bone Marrow Transplantation (2022)
-
Lineage-specific early complete donor chimerism and risk of relapse after allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia
Bone Marrow Transplantation (2022)
-
Intensive monitoring of minimal residual disease and chimerism after allogeneic hematopoietic stem cell transplantation for acute leukemia in children
Bone Marrow Transplantation (2021)