Abstract
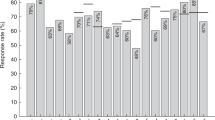
There have been no recommendations for revaccination with the Japanese encephalitis (JE) vaccine in post-hematopoietic stem cell transplantation (HSCT) patients. This study aimed to measure the immunogenic response to a live-attenuated JE vaccine (SA 14-14-2) in post-HSCT patients. JE-specific neutralizing Ab titers were measured before and after the JE vaccination. The patients with Ab titers <10 at the 3-month time point received a second injection at 6 months. A total of 28 patients (male:female=11:17) with a median age of 13 years (4–21 years) were included. The underlying diseases were thalassemia (50%) and hematologic malignancies (50%). Ten patients (35.7%) had Ab titers above the preventive level before vaccination. Nine of 18 patients (50%) seroconverted at 3 months after a single JE vaccination, but only three of these patients had sustained protective Ab levels. Seven of nine patients (78%) seroconverted at 3 months after a second JE vaccine injection, and all of these patients sustained protective Ab levels at 12 months. In conclusion, post-HSCT patients had low seroconversion rates after a single dose of the live-attenuated JE vaccine. These patients may require at least two doses of the JE vaccine to ensure protective Ab levels.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM et al. Estimated global incidence of Japanese encephalitis: a systematic review. Bull World Health Organ 2011; 89: 766–774E.
Trung NHD, Phuong TLT, Wolbers M, Minh HNV, Thanh VN, Van MP et al. Aetiologies of central nervous system infection in Viet Nam: A prospective provincial hospital-based descriptive surveillance study. PLoS ONE 2012; 7: e37825.
Rayamajhi A, Ansari I, Ledger E, Bista KP, Impoinvil DE, Nightingale S et al. Clinical and prognostic features among children with acute encephalitis syndrome in Nepal; a retrospective study. BMC infectious diseases 2011; 11: 294.
Upreti SR, Janusz KB, Schluter WW, Bichha P, Shakya G, Biggerstaff BJ et al. Estimation of the impact of a Japanese encephalitis immunization program with live, attenuated SA14-14-2 vaccine in Nepal. Am J Trop Med Hyg 2013; 88: 464–468.
Siraprapasiri T, Sawaddiwudhipong W, Rojanasuphot S . Cost benefit analysis of Japanese encephalitis vaccination program in Thailand. Southeast Asian J Trop Med Public Health 1997; 28: 143–148.
Touch S, Suraratdecha C, Samnang C, Heng S, Gazley L, Huch C et al. A cost-effectiveness analysis of Japanese encephalitis vaccine in Cambodia. Vaccine 2010; 28: 4593–4599.
Yin Z, Asay GRB, Zhang L, Li Y, Zuo S, Hutin YJ et al. An economic evaluation of the use of Japanese encephalitis vaccine in the expanded program of immunization of Guizhou province, China. Vaccine 30: 5569–5577.
World Health Organization. Japanese encephalitis surveillance and immunization-Asia and the western Pacific, 2012. MMWR 2013; 62: 658–662.
Xin YY, Ming ZG, Peng GY, Jian A, Min LH . Safety of a live-attenuated Japanese encephalitis virus vaccine (SA 14-14-2) for children. Am J Trop Med Hyg 1988; 39: 214–217.
Hennessy S, Liu Z, Tsai TF, Strom BL, Wan CM, Liu HL et al. Effectiveness of live-attenuated Japanese encephalitis vaccine (SA 14-14-2): a case-control study. Lancet 1996; 347: 1583–1586.
Tsai TF, Xin YY, Li JL, Putvatana R, Ran Z, Shougui W et al. Immunogenecity of live attenuated SA 14-14-2 Japanese encephalitis vaccine–a comparison of 1- and 3- month immunization schedules. J Infect Dis 1998; 177: 221–223.
Chotpitayasunondh T, Sohn YM, Yoksan S, Min J, Ohrr H . Immunizing children afed 9 to 15 months with live attenuated SA 14-14-2 Japanese encephalitis vaccine in Thailand. J Med Assoc Thai 2011; 94: S195–S203.
Liu ZL, Hennessy S, Strom BL, Tsai TF, Wan CM, Tang SC et al. Short-term safety of live attenuated Japanese encephalitis vaccine (14-14-2): results of a randomized trial with 26,239 subjects. J Infect Dis 1997; 176: 1366–1369.
Ljungman P, Lewensohn-Fuchs I, Hammarstrom V, Aschan J, Brandt L, Bolme P et al. Long-term immunity to measles, mumps, and rubella after allogeneic bone marrow transplantation. Blood 1994; 84: 657–663.
Inaba H, Hartford CM, Pei D, Posner MJ, Yang J, Hayden RT et al. Longitudinal analysis of antibody response to immunization in paediatric survivors after allogeneic haematopoietic stem cell transplantation. Br J Hematol 2011; 156: 109–117.
Ljungman P, Cordonnier C, Einsele H, Englund J, Machado CM, Storek J et al. Vaccination of hematopoietic cell transplant recipients. Bone Marrow Transplant 2009; 44: 521–526.
Russell PK, Nisalak A, Sukhavachana P, Vinona S . A plaque reduction test for dengue virus neutralizing antibodies. J Immunol 1967; 99: 285–290.
Centers for disease control and prevention. Japanese encephalitis vaccines: Recommendations of the advisory committee on immunization practices (ACIP). MMWR 2010; 59: 1–27.
Chokephaibulkit K, Plipat N, Yoksan S, Phongsamart W, Lappra K, Chearskul P et al. A comparative study of the serological response to Japanese encephalitis vaccine in HIV-infected and uninfected Thai children. Vaccine 2010; 28: 3563–3566.
Yang SE, Pan MJ, Tseng HF, Liau MY . The efficacy of mouse-brain inactivated Nakayama strain Japanese encephalitis vaccine–results from 30 years experience in Taiwan. Vaccine 2006; 24: 2669–2673.
Lindsey NP, Staples JE, Jones JF, Sejvar JJ, Griggs A, Iskander J et al. Adverse event reports following Japanese encephalitis vaccination in the United States, 1999–2009. Vaccine 2011; 29: 58–64.
Ohrr H, Tandam JB, Sohn YM, Pradhan DP . Halstead. Effect of single dose of SA 14-14-2 in Nepalese children with Japanese encephalitis: a case-control study. Lancet 2005; 366: 1375–1378.
Tandan JB, Ohrr H, Sohn YM, Yoksan S, Ji M, Nam CM et al. Single dose of SA 14-14-2 vaccine provides long-term protection against Japanese encephalitis: A case-control study in Nepalese children 5 years after immunization. Vaccine 2007; 25: 5041–5045.
Hau M, Schwartz KL, Frenette C, Mogck I, Gubbay JB, Severini A et al. Local public health response to vaccine-associated measles: case report. BMC Public Health 2013; 13: 269.
Kussmaul SC, Horn BN, Dvorak CC, Abramovitz L, Cowan MJ, Weintrub PS . Safety of the live, attenuated varicella vaccine in pediatric recipients of hematopoietic SCTs. Bone Marrow Transplant 2010; 45: 1602–1606.
Chou JF, Kernan NA, Prockop S, Heller G, Scaradavou A, Kobos R et al. Safety and immunogenicity of the live attenuated varicella vaccine following T replete or T cell-depleted related and unrelated allogeneic hematopoietic cell transplantation (alloHCT). Biol Blood Marrow Transplant 2011; 17: 1708–1713.
Acknowledgements
We thank Biogenetech for supplying the JE vaccine (SA14-14-2). SP received a grant from the Thai Hematology Society.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Pakakasama, S., Wattanatitan, S., Techasaensiri, C. et al. Immunogenicity of a live-attenuated Japanese encephalitis vaccine in children and adolescents after hematopoietic stem cell transplantation. Bone Marrow Transplant 49, 1307–1309 (2014). https://doi.org/10.1038/bmt.2014.149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2014.149
This article is cited by
-
Live Virus Vaccines in Transplantation: Friend or Foe?
Current Infectious Disease Reports (2015)