Abstract
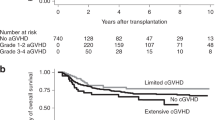
We studied whether early CsA trough levels were associated with the risk of acute GVHD in 337 patients after either sibling PBSC or double umbilical cord blood transplantation. All patients, regardless of donor type, started CsA at a dose of 5 mg/kg i.v. divided twice daily, targeting trough concentrations 200–400 ng/mL. The CsA level was studied by a weighted average method calculated by giving 70% of the weight to the level that was measured just before the onset of the event or day +30. We found that higher weighted average CsA trough levels early post transplantation contributed to lower risk of acute GVHD, and lower non-relapse and overall mortality. Thus, our data support close monitoring with active adjustments of CsA dosing to maintain therapeutic CsA levels in the first weeks of allo-HCT. In patients who are near or even modestly above the CsA target trough level, in the absence of CsA-related toxicity, dose reduction should be cautious to avoid subtherapeutic drug levels resulting in higher risk of acute GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Billingham RE, Brent L . A simple method for inducing tolerance of skin homografts in mice. Transplant Bull 1957; 4: 67–71.
Yee GC, Self SG, McGuire TR, Carlin J, Sanders JE, Deeg HJ . Serum cyclosporine concentration and risk of acute graft-versus-host disease after allogeneic marrow transplantation. N Engl J Med 1988; 319: 65–70.
Lamparelli T, Van Lint MT, Gualandi F, Occhini D, Barbanti M, Sacchi N et al. Bone marrow transplantation for chronic myeloid leukemia (CML) from unrelated and sibling donors: single center experience. Bone Marrow Transplant 1997; 20: 1057–1062.
Kedmi M, Dray L, Grisariu S, Resnick IB, Stepensky P, Aker M et al. The effect of cyclosporine initiation time on the outcome of matched allogeneic stem-cell transplantation following fludarabine-based conditioning. Transpl Int 2012; 25: 1241–1247.
Ram R, Storer B, Mielcarek M, Sandmaier BM, Maloney DG, Martin PJ et al. Association between calcineurin inhibitor blood concentrations and outcomes after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 414–422.
Malard F, Szydlo RM, Brissot E, Chevallier P, Guillaume T, Delaunay J et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2010; 16: 28–34.
Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood 2007; 110: 3064–3070.
Barker JN, Weisdorf DJ, DeFor TE, Blazar BR, McGlave PB, Miller JS et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005; 105: 1343–1347.
Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA et al. Allogeneic hematopoietic cell transplantation for hematological malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010; 116: 4693–4699.
Warlick E, Ahn KW, Pedersen TL, Artz A, de Lima M, Pulsipher M et al. Reduced intensity conditioning is superior to nonmyeloablative conditioning for older chronic myelogenous leukemia patients undergoing hematopoietic cell transplant during the tyrosine kinase inhibitor era. Blood 2012; 119: 4083–4090.
Adkins JC, Peters DH, Markham A . Fludarabine. An update of its pharmacology and use in the treatment of haematological malignancies. Drugs 1997; 53: 1005–1037.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Cox DR . Regression models and life tables. J Royal Stat Soc B. 1972; 34: 187–220.
Prentice RL, Williams BJ, Peterson AV . On the regression analysis of multivariate failure time data. Biometrika 1981; 68: 373–379.
Tarone RE, Ware J . Tests for trend in life table analysis. Biometrika 1975; 62: 679–687.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Blazar BR, Murphy WJ, Abedi M . Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol 2012; 12: 443–458.
MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood 2009; 113: 2410–2415.
Acknowledgements
This work was supported in part by grants from the National Cancer Institute PO1-CA65493 (CGB, BRB and TED) and Leukemia and Lymphoma Society Scholar in Clinical Research Award (CGB).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of intererst.
Rights and permissions
About this article
Cite this article
Rogosheske, J., Fargen, A., DeFor, T. et al. Higher therapeutic CsA levels early post transplantation reduce risk of acute GVHD and improves survival. Bone Marrow Transplant 49, 122–125 (2014). https://doi.org/10.1038/bmt.2013.139
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2013.139
Keywords
This article is cited by
-
Higher cyclosporine-A concentration increases the risk of relapse in AML following allogeneic stem cell transplantation from unrelated donors using anti-thymocyte globulin
Scientific Reports (2023)
-
Optimized cyclosporine starting dose may reduce risk of acute GvHD after allogeneic hematopoietic cell transplantation: a single-center cohort study
Bone Marrow Transplantation (2022)
-
Severity of mucositis during allogeneic transplantation impacts post-transplant cyclosporin absorption
Bone Marrow Transplantation (2020)
-
Cyclosporine levels > 195 μg/L on day 10 post-transplant was associated with significantly reduced acute graft-versus-host disease following allogeneic hematopoietic stem cell transplantation
Annals of Hematology (2019)
-
Elevated bone marrow eosinophil count is associated with high incidence of severe acute GvHD after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2017)