Abstract
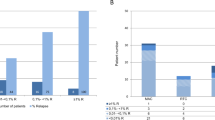
Estimation of relapse risk in AML after allo-SCT is critical. The negative impact of increased blast count post transplant is widely accepted. Here, we studied cellularity and dysplasia in BM cytomorphology on days 30 and 100 in 112 AML patients who achieved haematological CR after SCT. Overall cellularity on day 30 was normal in 45.3%, reduced in 37.3% and increased in 17.3% of samples (day 100: normal: 54.8%; reduced: 38.7%; and increased: 6.5%). Dysplasia in ⩾10% of cells was frequent on day 30 (granulopoiesis: 25.0% of samples; erythropoiesis: 34.6%; and megakaryopoiesis: 47.7%) and also on day 100. Relapses were less frequent in patients with normal BM cellularity on day 30 (7/34; 20.6%) when compared with reduced (9/28; 32.1%) or increased cellularity (10/13; 76.9%; P=0.001). Estimated 2-year OS was 59.0% for patients with normal overall cellularity, followed by patients with increased (44.0%) and reduced cellularity (31.4%, P=0.009). In contrast, cellularity at day 100 and dysplasia at days 30 and 100 did not correlate with outcome measures. Thus, in the cohort studied, BM cellularity represents a prognostic parameter for the post-transplant period in AML patients. Dysplasia seems to be an unspecific phenomenon in the cohort analysed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Armand P, Kim HT, DeAngelo DJ, Ho VT, Cutler CS, Stone RM et al. Impact of cytogenetics on outcome of de novo and therapy-related AML and MDS after allogeneic transplantation. Biol Blood Marrow Transplant 2007; 13: 655–664.
Kröger N, Bacher U, Bader P, Bottcher S, Borowitz MJ, Dreger P et al. NCI first international workshop on the biology, prevention, and treatment of relapse after allogeneic hematopoietic stem cell transplantation: report from the committee on disease-specific methods and strategies for monitoring relapse following allogeneic stem cell transplantation. Part I: methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant 2010; 16: 1187–1211.
Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 1999; 13: 2079–2086.
Platzbecker U, Wermke M, Radke J, Oelschlaegel U, Seltmann F, Kiani A et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial. Leukemia 2011; 26: 381–389.
Sockel K, Wermke M, Radke J, Kiani A, Schaich M, Bornhauser M et al. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica 2011; 96: 1568–1570.
Hokland P, Ommen HB . Towards individualized follow-up in adult acute myeloid leukemia in remission. Blood 2011; 117: 2577–2584.
Bacher U, Badbaran A, Fehse B, Zabelina T, Zander AR, Kröger N . Quantitative monitoring of NPM1 mutations provides a valid minimal residual disease parameter following allogeneic stem cell transplantation. Exp Hematol 2009; 37: 135–142.
Bacher U, Haferlach T, Fehse B, Schnittger S, Kröger N . Minimal residual disease diagnostics and chimerism in the post-transplant period in acute myeloid leukemia. ScientificWorldJournal 2011; 11: 310–319.
Stahl T, Badbaran A, Kröger N, Klyuchnikov E, Zabelina T, Zeschke S et al. Minimal residual disease diagnostics in patients with acute myeloid leukemia in the post-transplant period: comparison of peripheral blood and bone marrow analysis. Leuk Lymphoma 2010; 51: 1837–1843.
Schnittger S, Kern W, Tschulik C, Weiss T, Dicker F, Falini B et al. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood 2009; 114: 2220–2231.
Dominietto A, Pozzi S, Miglino M, Albarracin F, Piaggio G, Bertolotti F et al. Donor lymphocyte infusions for the treatment of minimal residual disease in acute leukemia. Blood 2007; 109: 5063–5064.
Rettinger E, Willasch AM, Kreyenberg H, Borkhardt A, Holter W, Kremens B et al. Preemptive immunotherapy in childhood acute myeloid leukemia for patients showing evidence of mixed chimerism after allogeneic stem cell transplantation. Blood 2011; 118: 5681–5688.
Lange T, Hubmann M, Burkhardt R, Franke GN, Cross M, Scholz M et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 2011; 25: 498–505.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 2003; 21: 4642–4649.
de Greef GE, van Putten WL, Boogaerts M, Huijgens PC, Verdonck LF, Vellenga E et al. Criteria for defining a complete remission in acute myeloid leukaemia revisited. An analysis of patients treated in HOVON-SAKK co-operative group studies. Br J Haematol 2005; 128: 184–191.
van Marion AM, Thiele J, Kvasnicka HM, van den Tweel JG . Morphology of the bone marrow after stem cell transplantation. Histopathology 2006; 48: 329–342.
Scott BL, Storer BE, Greene JE, Hackman RC, Appelbaum FR, Deeg HJ . Marrow fibrosis as a risk factor for posttransplantation outcome in patients with advanced myelodysplastic syndrome or acute myeloid leukemia with multilineage dysplasia. Biol Blood Marrow Transplant 2007; 13: 345–354.
Tuzuner N, Bennett JM . Reference standards for bone marrow cellularity. Leuk Res 1994; 18: 645–647.
Tuzuner N, Cox C, Rowe JM, Bennett JM . Bone marrow cellularity in myeloid stem cell disorders: impact of age correction. Leuk Res 1994; 18: 559–564.
Goasguen JE, Matsuo T, Cox C, Bennett JM . Evaluation of the dysmyelopoiesis in 336 patients with de novo acute myeloid leukemia: major importance of dysgranulopoiesis for remission and survival. Leukemia 1992; 6: 520–525.
Brunning RD, Orazi A, Germing U, Le Beau MM, Porwit A, Baumann I et al. Myelodysplastic syndromes/neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL eds.. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues 4th Edn. IARC Press Lyon, France, 2008, 88–93.
Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010; 116: 354–365.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002; 99: 4618–4625.
Fehse B, Chukhlovin A, Kuhlcke K, Marinetz O, Vorwig O, Renges H et al. Real-time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J Hematother Stem Cell Res 2001; 10: 419–425.
Kaplan EL, Meier P . Nonparametric Estimation from Incomplete Observations. J Am Stat Assoc 1958; 53: 457–481.
Gray RJA . Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat 1988; 16: 1141–1154.
Cox DR . Regression models and live tables. J R Stat Soc B 1972; 34: 187–220.
Fine JP, Gray RJ . A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Scrucca L, Santucci A, Aversa F . Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant 2010; 45: 1388–1395.
Scrucca L, Santucci A, Aversa F . Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant 2007; 40: 381–387.
Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ . Sequential regimen of chemotherapy, reduced-intensity conditioning for allogeneic stem-cell transplantation, and prophylactic donor lymphocyte transfusion in high-risk acute myeloid leukemia and myelodysplastic syndrome. J Clin Oncol 2005; 23: 5675–5687.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Christopeit M, Feldmann U, Finke J, Bunjes DW, Beelen DW, Bornhaeuser M et al. Shift to a new donor does not improve the outcome after second allogeneic stem cell transplantation (alloSCT) in acute leukemia relapse after a first allosct - a risk factor analysis by the German Stem Cell Registry (DRST). ASH Annual Meeting Abstracts 2009; 114: 3328.
Chakrabarti S, Brown J, Guttridge M, Pamphilon DH, Lankester A, Marks DI . Early lymphocyte recovery is an important determinant of outcome following allogeneic transplantation with CD34+ selected graft and limited T-cell addback. Bone Marrow Transplant 2003; 32: 23–30.
Le Blanc K, Barrett AJ, Schaffer M, Hagglund H, Ljungman P, Ringden O et al. Lymphocyte recovery is a major determinant of outcome after matched unrelated myeloablative transplantation for myelogenous malignancies. Biol Blood Marrow Transplant 2009; 15: 1108–1115.
Savani BN, Mielke S, Rezvani K, Montero A, Yong AS, Wish L et al. Absolute lymphocyte count on day 30 is a surrogate for robust hematopoietic recovery and strongly predicts outcome after T cell-depleted allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2007; 13: 1216–1223.
Barragan E, Pajuelo JC, Ballester S, Fuster O, Cervera J, Moscardo F et al. Minimal residual disease detection in acute myeloid leukemia by mutant nucleophosmin (NPM1): comparison with WT1 gene expression. Clin Chim Acta 2008; 395: 120–123.
Ommen HB, Schnittger S, Jovanovic JV, Ommen IB, Hasle H, Ostergaard M et al. Strikingly different molecular relapse kinetics in NPM1c, PML-RARA, RUNX1-RUNX1T1, and CBFB-MYH11 acute myeloid leukemias. Blood 2010; 115: 198–205.
Inoue K, Sugiyama H, Ogawa H, Nakagawa M, Yamagami T, Miwa H et al. WT1 as a new prognostic factor and a new marker for the detection of minimal residual disease in acute leukemia. Blood 1994; 84: 3071–3079.
Cilloni D, Renneville A, Hermitte F, Hills RK, Daly S, Jovanovic JV et al. Real-time quantitative polymerase chain reaction detection of minimal residual disease by standardized WT1 assay to enhance risk stratification in acute myeloid leukemia: a European LeukemiaNet study. J Clin Oncol 2009; 27: 5195–5201.
Zeiser R, Spyridonidis A, Wasch R, Ihorst G, Grullich C, Bertz H et al. Evaluation of immunomodulatory treatment based on conventional and lineage-specific chimerism analysis in patients with myeloid malignancies after myeloablative allogeneic hematopoietic cell transplantation. Leukemia 2005; 19: 814–821.
Mattsson J, Uzunel M, Tammik L, Aschan J, Ringden O . Leukemia lineage-specific chimerism analysis is a sensitive predictor of relapse in patients with acute myeloid leukemia and myelodysplastic syndrome after allogeneic stem cell transplantation. Leukemia 2001; 15: 1976–1985.
Bornhäuser M, Oelschlaegel U, Platzbecker U, Bug G, Lutterbeck K, Kiehl MG et al. Monitoring of donor chimerism in sorted CD34+ peripheral blood cells allows the sensitive detection of imminent relapse after allogeneic stem cell transplantation. Haematologica 2009; 94: 1613–1617.
de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K et al. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer 2010; 116: 5420–5431.
Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P et al. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer 2009; 115: 1899–1905.
Graef T, Kuendgen A, Fenk R, Zohren F, Haas R, Kobbe G . Successful treatment of relapsed AML after allogeneic stem cell transplantation with azacitidine. Leuk Res 2007; 31: 257–259.
Acknowledgements
We acknowledge Waltraud Schultz, University Cancer Centre Hamburg, for expert technical assistance (cytomorphology). We also acknowledge Dr Michael Roth MD, Department of Pediatrics, Montefiore Hospital and Department of Cell Biology, Albert Einstein College of Medicine, Bronx, NY, USA, for critical comments, thoughtful suggestions on data interpretation and critical reading of the manuscript.
Author contributions: MC and UB designed the study and wrote the manuscript. MC, KM, MB, PS and UB performed cytomorphology. KM, TZ, MB and UB assembled data. MC, EK and UB carried out statistical analysis. FA, CB and NK contributed patients. TH and NK contributed to the interpretation of data. All authors contributed to data analysis, reviewed the manuscript and approved its final version.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Data have been assembled during the course of the doctoral thesis of KM. MC has received honoraria and travel support from Celgene. TH declares part ownership of the Munich Leukemia Laboratory GmbH. All other authors have no conflict of interest to declare.
Additional information
Supplementary Information accompanies the paper on Bone Marrow Transplantation website
Rights and permissions
About this article
Cite this article
Christopeit, M., Miersch, K., Klyuchnikov, E. et al. Evaluation of BM cytomorphology after allo-SCT in patients with AML. Bone Marrow Transplant 47, 1538–1544 (2012). https://doi.org/10.1038/bmt.2012.70
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.70
Keywords
This article is cited by
-
Relapse assessment following allogeneic SCT in patients with MDS and AML
Annals of Hematology (2014)
-
Multilineage dysplasia is associated with a poorer prognosis in patients with de novo acute myeloid leukemia with intermediate-risk cytogenetics and wild-type NPM1
Annals of Hematology (2014)
-
Evaluation of BM cytomorphology after allo-SCT in patients with MDS
Bone Marrow Transplantation (2013)
-
Impact of physiological BM CD10+CD19+ B-cell precursors (haematogones) in the post-transplant period in patients with AML
Bone Marrow Transplantation (2013)