Abstract
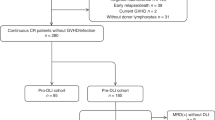
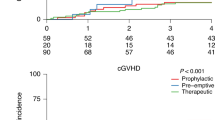
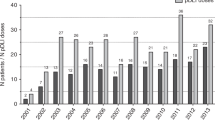
Peripheral blood used as a source of stem cells for transplantation (PBSCT) is known to exert stronger immune-mediated effects compared with BM (BMT). We decided to retrospectively analyze the impact of stem cell source on the OS of CML patients who relapsed after either matched related donor PBSCT (N=168) or BMT (N=216) and were treated with donor lymphocyte infusions (DLI). Univariate analysis revealed a lower probability of OS after DLI in patients relapsing after PBSCT vs BMT (66% vs 79% at 5 years, P=0.013). However, a multivariate Cox analysis did not reveal any significant impact of PBSCT as a risk factor for decreased OS for patients transplanted in first chronic phase (CP1; hazard ratio (HR) 1.036, 95% confidence interval (CI) 0.619–1.734). A statistical interaction term suggested that the impact of stem cell source on OS after DLI was different for those transplanted in advanced phases (negative impact of previous PBSCT—HR 2.176, 95% CI 0.930–5.091). In summary, the stem cell source does not affect the OS of CML patients who underwent PBSCT in CP1, relapsed and were treated with DLI. However, when the patients were transplanted in advanced phases, previous PBSCT seems to negatively affect OS after DLI compared with BMT.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Gratwohl A, Heim D . Current role of stem cell transplantation in chronic myeloid leukaemia. Best Pract Res Clin Haematol 2009; 22: 431–443.
Baldomero H, Gratwohl M, Gratwohl A, Tichelli A, Niederwieser D, Madrigal A et al. The EBMT activity survey 2009: trends over the past 5 years. Bone Marrow Transplant 2011; 46: 485–501.
Stem Cell Trialists’ Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol 2005; 23: 5074–5087.
Schmitz N, Eapen M, Horowitz MM, Zhang MJ, Klein JP, Rizzo JD et al. Long-term outcome of patients given transplants of mobilized blood or bone marrow: a report from the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. Blood 2006; 108: 4288–4290.
Eapen M, Logan BR, Confer DL, Haagenson M, Wagner JE, Weisdorf DJ et al. Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant 2007; 13: 1461–1468.
Guglielmi C, Arcese W, Dazzi F, Brand R, Bunjes D, Verdonck LF et al. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose. Blood 2002; 100: 397–405.
Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol 1997; 15: 433–444.
Alyea EP, Soiffer RJ, Canning C, Neuberg D, Schlossman R, Pickett C et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood 1998; 91: 3671–3680.
Dazzi F, Hasserjian R, Gordon MY, Boecklin F, Cotter F, Corbo M et al. Normal and chronic phase CML hematopoietic cells repopulate NOD/SCID bone marrow with different kinetics and cell lineage representation. Hematol J 2000; 1: 307–315.
Elmaagacli AH, Basoglu S, Peceny R, Trenschel R, Ottinger H, Lollert A et al. Improved disease-free-survival after transplantation of peripheral blood stem cells as compared with bone marrow from HLA-identical unrelated donors in patients with first chronic phase chronic myeloid leukemia. Blood 2002; 99: 1130–1135.
Carlens S, Remberger M, Aschan J, Ringden O . The role of disease stage in the response to donor lymphocyte infusions as treatment for leukemic relapse. Biol Blood Marrow Transplant 2001; 7: 31–38.
Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000; 96: 2712–2716.
Raiola AM, Van Lint MT, Valbonesi M, Lamparelli T, Gualandi F, Occhini D et al. Factors predicting response and graft-versus-host disease after donor lymphocyte infusions: a study on 593 infusions. Bone Marrow Transplant 2003; 31: 687–693.
Kolb HJ, Schmid C, Buhmann R, Tischer J, Ledderose G . DLI: where are we know? Hematology 2005; 10 (Suppl 1): 115–116.
Simula MP, Marktel S, Fozza C, Kaeda J, Szydlo RM, Nadal E et al. Response to donor lymphocyte infusions for chronic myeloid leukemia is dose-dependent: the importance of escalating the cell dose to maximize therapeutic efficacy. Leukemia 2007; 21: 943–948.
Speck B, Bortin MM, Champlin R, Goldman JM, Herzig RH, McGlave PB et al. Allogeneic bone-marrow transplantation for chronic myelogenous leukaemia. Lancet 1984; 1: 665–668.
Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant 2010; 45: 558–564.
Matthews DE, Farewell VT . Using and Understanding Medical Statistics. Karger: Basel, 1996.
European Group for Blood and Marrow Transplantation Internet access: ProMISe. Available from www.ebmt.org/Contents/Data-Management/Registrystructure/ DatamanagementsystemProMIse/Pages/Data-management-system-ProMIse.aspx (Accessed on 21 September 2012).
Savani BN, Montero A, Kurlander R, Childs R, Hensel N, Barrett AJ . Imatinib synergizes with donor lymphocyte infusions to achieve rapid molecular remission of CML relapsing after allogeneic stem cell transplantation. Bone Marrow Transplant 2005; 36: 1009–1015.
Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY et al. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol 2010; 11: 331–338.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Basak, G., de Wreede, L., van Biezen, A. et al. Donor lymphocyte infusions for the treatment of chronic myeloid leukemia relapse following peripheral blood or bone marrow stem cell transplantation. Bone Marrow Transplant 48, 837–842 (2013). https://doi.org/10.1038/bmt.2012.234
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.234
Keywords
This article is cited by
-
Escalating-dose HLA-mismatched DLI is safe for the treatment of leukaemia relapse following alemtuzumab-based myeloablative allo-SCT
Bone Marrow Transplantation (2013)