Abstract
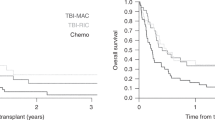
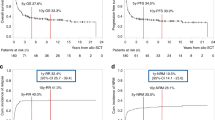
For patients with ALL who relapse following allo-SCT, only a second SCT provides a realistic chance for long-term disease remission. We retrospectively analyzed the outcomes of 31 patients with relapsed ALL after a prior allo-SCT, who received a second SCT (SCT2) at our center. With a median follow-up of 3 years, 1- and 3-year PFS was 23 and 11% and 1- and 3 year OS rates were 23 and 11%. Twelve patients (39%) were transplanted with active disease, of whom 75% attained a CR. We found a significant relationship between the time to treatment failure following first allograft (SCT1) and PFS following SCT2 (P=0.02, hazard ratio=0.93/month). In summary, a second transplant remains a potential treatment option for achieving response in a highly refractory patient population. While long-term survival is limited, a significant proportion of patients remains disease-free for up to 1 year following SCT2, providing a window of time to administer preventive interventions. Notably, our four long-term survivors received novel therapies with their second transplant underscoring the need for a fundamental change in the methods for SCT2 to improve outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Spyridonidis A, Labopin M, Schmid C, Volin L, Yakoub-Agha I, Stadler M et al. Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia 2012; 26: 1211–1217.
Fielding AK, Richards SM, Chopra R, Lazarus HM, Litzow MR, Buck G et al. Outcome of 609 adults after relapse of acute lymphoblastic leukemia (ALL); an MRC UKALL12/ECOG 2993 study. Blood 2007; 109: 944–950.
Eapen M, Giralt SA, Horowitz MM, Klein JP, Wagner JE, Zhang MJ et al. Second transplant for acute and chronic leukemia relapsing after first HLA-identical sibling transplant. Bone Marrow Transplant 2004; 34: 721–727.
Bosi A, Laszlo D, Labopin M, Reffeirs J, Michallet M, Gluckman E et al. Second allogeneic bone marrow transplantation in acute leukemia: results of a survey by the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol 2001; 19: 3675–3684.
Moorman AV, Harrison CJ, Buck GA, Richards SM, Secker-Walker LM, Martineau M et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALLXII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood 2007; 109: 3189–3197.
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 2009; 15: 1628–1633.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB et al. Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981; 57: 267–276.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Jason PF, Gray RJA . Proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999; 94: 496–509.
Kantarjian HM, O'Brien S, Smith TL, Cortes J, Giles FJ, Beran M et al. Results of treatment with hyper-CVAD, a dose-intensive regimen, in adult acute lymphocytic leukemia. J Clin Oncol 2000; 18: 547–561.
Bosi A, Bacci S, Miniero R, Locatelli F, Laszlo D, Longo G et al. Second allogeneic bone marrow transplantation in acute leukemia: a multicenter study from the Gruppo Italiano Trapianto Di Midollo Osseo (GITMO). Leukemia 1997; 11: 420–424.
Mack M, Riethmuller G, Kufer P . A small bispecific antibody construct expressed as a functional single-chain molecule with high tumor cell cytotoxicity. Proc Natl Acad Sci 1995; 92: 7021–7025.
Loffler A, Kufer P, Lutterbuse R, Zettl F, Daniel PT, Schwenkenbecher JM et al. A recombinant bispecific single-chain antibody, CD19XCD3, induces rapid and high lymphoma-directed cytotoxicity by unstimulated T lymphocytes. Blood 2000; 95: 2098–2103.
Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S et al. Targeted therapy with the T-Cell engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in b-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29: 2493–2498.
Kantarjian H, Thomas D, Jorgensen J, Jabbour E, Kebriaei P, Rytting M et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol 13: 403–411.
Topp MS, Goekbuget N, Zugmaier G, Viardot A, Stelljes M, Neumann S et al. Anti-CD19 BiTE blinatumomab induces high complete remission rate in adult patients with relapsed B-precursor ALL:Updated Results of An Ongoing Phase II Trial. ASH Annual Meeting Abstracts 118: 252.
Jena B, Dotti G, Cooper LJ . Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010; 116: 1035–1044.
Kebriaei P, Kelly SS, Manuri P, Jena B, Jackson R, Shpall E et al. CARs: driving T-cell specificity to enhance anti-tumor immunity. Front Biosci (Schol Ed) 2012; 4: 520–531.
Park JH, Sauter C, Brentjens R . Cellular therapies in acute lymphoblastic leukemia. Hematol Oncol Clin North Am 2011; 25: 1281–1301.
Kishi K, Takahashi S, Gondo H, Shiobara S, Kanamaru A, Kato S et al. Second allogeneic bone marrow transplantation for post-transplant leukemia relapse: results of a survey of 66 cases in 24 Japanese institutes. Bone Marrow Transplant 1997; 19: 461–466.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Poon, L., Bassett Jr, R., Rondon, G. et al. Outcomes of second allogeneic hematopoietic stem cell transplantation for patients with acute lymphoblastic leukemia. Bone Marrow Transplant 48, 666–670 (2013). https://doi.org/10.1038/bmt.2012.195
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2012.195
Keywords
This article is cited by
-
Outcomes in patients with acute lymphoblastic leukemia who underwent second allogeneic hematopoietic cell transplantation for relapse after first transplantation
International Journal of Hematology (2022)
-
Efficacy and safety of CD19 CAR-T cell therapy for acute lymphoblastic leukemia patients relapsed after allogeneic hematopoietic stem cell transplantation
International Journal of Hematology (2022)
-
Donor-derived anti-CD19 CAR T cells compared with donor lymphocyte infusion for recurrent B-ALL after allogeneic hematopoietic stem cell transplantation
Bone Marrow Transplantation (2021)
-
Outcome predictors after retransplantation in relapsed acute lymphoblastic leukemia: a multicenter, retrospective study
Annals of Hematology (2021)
-
Donor-derived CD19 CAR-T cell therapy of relapse of CD19-positive B-ALL post allotransplant
Leukemia (2021)