Abstract
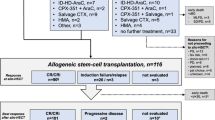
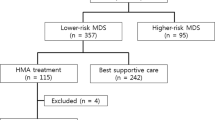
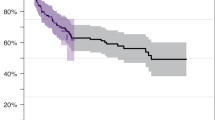
The role of hypomethylating agent therapy (HMT) as a bridge to allogeneic hematopoietic cell transplantation (alloHCT) in patients with myelodysplastic syndrome (MDS) remains undetermined. We investigated the feasibility of HMT followed by alloHCT in patients with MDS. In all, 19 patients who received HMT followed by alloHCT were analyzed. A total of 7 patients were classified as low-risk and 12 as high-risk, based on World Health Organization (WHO) classification at the time of HMT. HMT consisted of decitabine in 9 patients and azacitidine in 10. After HMT, two patients achieved CR, six mCR, three hematologic improvement alone, and six SD in terms of best response. HMT did not alter WHO classification in 15 patients (79%), whereas 1 patient (5%) improved and 3 (16%) progressed to AML. Most patients (95%) received a non-myeloablative conditioning regimen based on fludarabine/BU/anti-thymocyte globulin, and peripheral blood-mobilized stem cells. Neutrophil and platelet engraftments were achieved in 95 and 79% of patients, respectively. The incidences of acute and chronic GVHD were 42 and 26%, respectively. In all, 2-year OS rates were 68%, and the overall outcomes of those who achieved CR/mCR with HMT tended to be superior to those without CR/mCR. HMT followed by alloHCT was a feasible and effective treatment strategy for patients with MDS.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kim DY, Lee JH, Lee JH, Lee KH, Kim YK, Ahn JS et al. Comparison of various criteria in predicting treatment response and prognosis of patients with myelodysplastic syndrome treated with azacitidine. Ann Hematol 2010; 89: 15–23.
Silverman LR, McKenzie DR, Peterson BL, Holland JF, Backstrom JT, Beach CL et al. Further analysis of trials with azacitidine in patients with myelodysplastic syndrome: studies 8421 8921 and 9221 by the Cancer and Leukemia Group B. J Clin Oncol 2006; 24: 3895–3903.
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232.
Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106: 1794–1803.
Wijermans P, Suciu S, Baila L, Platzbecker U, Giagounidis A, Selleslag D et al. low dose decitabine versus best supportive care in elderly patients with intermediate or high risk MDS not eligible for intensive chemotherapy: final results of the randomized Phase III Study (06011) of the EORTC Leukemia and German MDS Study Groups. Blood 2008; 112: 90.
Kantarjian H, Oki Y, Garcia-Manero G, Huang X, O’Brien S, Cortes J et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood 2007; 109: 52–57.
Wijermans P, Lubbert M, Verhoef G, Bosly A, Ravoet C, Andre M et al. Low-dose 5-aza-2′-deoxycytidine, a DNA hypomethylating agent, for the treatment of high-risk myelodysplastic syndrome: a multicenter phase II study in elderly patients. J Clin Oncol 2000; 18: 956–962.
Wijermans PW, Krulder JW, Huijgens PC, Neve P . Continuous infusion of low-dose 5-Aza-2′-deoxycytidine in elderly patients with high-risk myelodysplastic syndrome. Leukemia 1997; 11: 1–5.
Steensma DP, Baer MR, Slack JL, Buckstein R, Godley LA, Garcia-Manero G et al. Multicenter study of decitabine administered daily for 5 days every 4 weeks to adults with myelodysplastic syndromes: the alternative dosing for outpatient treatment (ADOPT) trial. J Clin Oncol 2009; 27: 3842–3848.
Raza A, Reeves JA, Feldman EJ, Dewald GW, Bennett JM, Deeg HJ et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 2008; 111: 86–93.
Melchert M, List A . Targeted therapies in myelodysplastic syndrome. Semin Hematol 2008; 45: 31–38.
Kindwall-Keller T, Isola LM . The evolution of hematopoietic SCT in myelodysplastic syndrome. Bone Marrow Transplant 2009; 43: 597–609.
Issa JP . Optimizing therapy with methylation inhibitors in myelodysplastic syndromes: dose, duration, and patient selection. Nat Clin Pract Oncol 2005; 2 (Suppl 1): S24–S29.
Toyota M, Kopecky KJ, Toyota MO, Jair KW, Willman CL, Issa JP . Methylation profiling in acute myeloid leukemia. Blood 2001; 97: 2823–2829.
Pinto A, Maio M, Attadia V, Zappacosta S, Cimino R . Modulation of HLA-DR antigens expression in human myeloid leukaemialeukemia cells by cytarabine and 5-aza-2′-deoxycytidine. Lancet 1984; 2: 867–868.
Pinto A, Zagonel V . 5-Aza-2′-deoxycytidine (Decitabine) and 5-azacytidine in the treatment of acute myeloid leukemias and myelodysplastic syndromes: past, present and future trends. Leukemia 1993; 7 (Suppl 1): 51–60.
Pinto A, Zagonel V, Attadia V, Bullian PL, Gattei V, Carbone A et al. 5-Aza-2′-deoxycytidine as a differentiation inducer in acute myeloid leukaemias and myelodysplastic syndromes of the elderly. Bone Marrow Transplant 1989; 4 (Suppl 3): 28–32.
Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 2000; 96: 3671–3674.
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005; 11: 945–956.
De Padua Silva L, de Lima M, Kantarjian H, Faderl S, Kebriaei P, Giralt S et al. Feasibility of allo-SCT after hypomethylating therapy with decitabine for myelodysplastic syndrome. Bone Marrow Transplant 2009; 43: 839–843.
Field T, Perkins J, Huang Y, Kharfan-Dabaja MA, Alsina M, Ayala E et al. 5-Azacitidine for myelodysplasia before allogeneic hematopoietic cell transplantation. Bone Marrow Transplant 2010; 45: 255–260.
Lubbert M, Bertz H, Ruter B, Marks R, Claus R, Wasch R et al. Non-intensive treatment with low-dose 5-aza-2′-deoxycytidine (DAC) prior to allogeneic blood SCT of older MDS/AML patients. Bone Marrow Transplant 2009; 44: 585–588.
Cogle CR, Imanirad I, Wiggins LE, Hsu J, Brown R, Scornik JC et al. Hypomethylating agent induction therapy followed by hematopoietic cell transplantation is feasible in patients with myelodysplastic syndromes. Clin Adv Hematol Oncol 2010; 8: 40–46.
Lee JH, Lee JH, Lim SN, Kim DY, Kim SH, Lee YS et al. Allogeneic hematopoietic cell transplantation for myelodysplastic syndrome: prognostic significance of pre-transplant IPSS score and comorbidity. Bone Marrow Transplant 2010; 45: 450–457.
Acknowledgements
This study was supported by a Grant (2010-489) from the Asan Institute for Life Sciences, Seoul, Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kim, DY., Lee, JH., Park, YH. et al. Feasibility of hypomethylating agents followed by allogeneic hematopoietic cell transplantation in patients with myelodysplastic syndrome. Bone Marrow Transplant 47, 374–379 (2012). https://doi.org/10.1038/bmt.2011.86
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.86
Keywords
This article is cited by
-
Increased opportunity for prolonged survival after allogeneic hematopoietic stem cell transplantation in patients aged 60–69 years with myelodysplastic syndrome
Annals of Hematology (2019)
-
Decitabine for relapsed acute lymphoblastic leukemia after allogeneic hematopoietic stem cell transplantation
Current Medical Science (2017)
-
Impact of prior azacitidine on the outcome of allogeneic hematopoietic transplantation for myelodysplastic syndrome
Pathology & Oncology Research (2015)
-
Azacitidine salvage therapy for relapse of myeloid malignancies following allogeneic hematopoietic SCT
Bone Marrow Transplantation (2014)
-
Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML
Bone Marrow Transplantation (2013)