Abstract
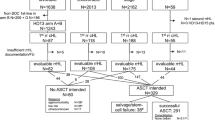
One challenge in designing clinical trials for treatment of acute GVHD (aGVHD) is the lack of an established standardized end point to measure the success of therapies. To facilitate assessment of end points in clinical trials for treatment of aGVHD in the current allo-SCT era, a national workshop was recently organized. In this study, which was presented at the workshop, we evaluated the prognostic value of response to upfront therapy in a cohort of 83 patients who had been enrolled on two clinical trials testing novel therapies for aGVHD at our institution. Our results indicate that patients whose aGVHD has a CR or PR by day 28 after initiation of systemic therapy have a significantly lower 6-month cumulative incidence of non-relapse mortality (NRM) (16%) than patients whose aGVHD did not respond to therapy by day 28 (48%, P=0.005). Multivariate analysis based on the Cox proportional hazards regression analysis showed that the impact of response on NRM is independent of patient and aGVHD characteristics. Our data confirm the validity of using day-28 response as a primary end point in clinical trials for upfront therapy for aGVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Martin PJ, Bachier CR, Klingemann HG, McCarthy PL, Szabolcs P, Uberti JP et al. Endpoints for clinical trials testing treatment of acute graft-versus-host disease: a joint statement. Biol Blood Marrow Transplant 2009; 15: 777–784.
Martin PJ, Nash RA . Pitfalls in the design of clinical trials for prevention or treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 2006; 12 (Suppl 2): 31–36.
Alousi AM, Weisdorf DJ, Logan BR, Bolanos-Meade J, Carter S, Difronzo N et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood 2009; 114: 511–517.
Couriel DR, Saliba R, de Lima M, Giralt S, Andersson B, Khouri I et al. A phase III study of infliximab and corticosteroids for the initial treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant 2009; 15: 1555–1562.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Akaike H . A new look at the statistical model identification. Transactions on Automatic Control 1974; 19: 716–723.
MacMillan ML, DeFor TE, Weisdorf DJ . The best endpoint for acute GVHD treatment trials. Blood 2010; 115: 5412–5417.
Levine JE, Logan B, Wu J, Alousi AM, Ho V, Bolanos-Meade J et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant 2010; 16: 1693–1699.
Cahn J-Y, Klein JP, Lee SJ, Milpied N, Blaise D, Antin JH et al. Prospective evaluation of 2 acute graft-versus-host (GVHD) grading systems: a joint Societe Francaise de Greffe de Moelle et Therapie Cellulaire (SFGM-TC), Dana Farber Cancer Institute (DFCI), and International Bone Marrow Transplant Registry (IBMTR) prospective study. Blood 2005; 106: 1495–1500.
Leisenring WM, Martin PJ, Petersdorf EW, Regan AE, Aboulhosn N, Stern JM et al. An acute graft-versus-host disease activity index to predict survival after hematopoietic cell transplantation with myeloablative conditioning regimens. Blood 2006; 108: 749–755.
MacMillan ML, Weisdorf DJ, Wagner JE, DeFor TE, Burns LJ, Ramsay NK et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant 2002; 8: 387–394.
Westin JR, Saliba RM, de Lima M, Alousi A, Hosing C, Qazilbash MH et al. Risk factors for response after initial therapy for acute graft-versus-host-disease (aGVHD). ASH Annu Meeting Abstracts 2007; 110: 5015.
Pasquini MC . Impact of graft-versus-host disease on survival. Best Pract Res Clin Haematol 2008; 21: 193–204.
Acknowledgements
We acknowledge our patients and clinical staff, without whom this research would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
The results of this work have been partially presented at an acute GVHD workshop in Bethesda, MD, in 2009.
Rights and permissions
About this article
Cite this article
Saliba, R., Couriel, D., Giralt, S. et al. Prognostic value of response after upfront therapy for acute GVHD. Bone Marrow Transplant 47, 125–131 (2012). https://doi.org/10.1038/bmt.2011.41
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.41
Keywords
This article is cited by
-
First-line steroid-free systemic treatment of acute and chronic graft-versus-host disease after novel prophylaxis regimens
Bone Marrow Transplantation (2023)
-
Current and Emerging Targeted Therapies for Acute Graft-Versus-Host Disease
BioDrugs (2021)
-
The new refined minnesota risk score for acute graft-versus-host disease predicts overall survival and non-relapse mortality after T cell-replete haploidentical stem cell transplant with post-transplant cyclophosphamide
Bone Marrow Transplantation (2019)
-
Evaluation of infliximab as second-line treatment of acute graft versus host disease -validating response on day 7 and 28 as predictors of survival
Bone Marrow Transplantation (2018)
-
Effective treatment of steroid and therapy-refractory acute graft-versus-host disease with a novel mesenchymal stromal cell product (MSC-FFM)
Bone Marrow Transplantation (2018)