Abstract
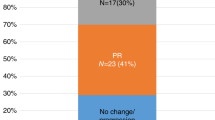
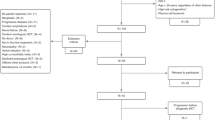
The relevance of high-dose chemotherapy followed by auto-SCT in CLL remains to be defined. The aim of the prospective, randomized, GOELAMS LLC 98 trial was to compare two strategies in previously untreated CLL patients aged <60 years. Conventional chemotherapy (Arm A) consisted of six monthly courses of CHOP followed by six CHOP courses in every 3 months in those achieving a complete or PR. Arm A was compared with high-dose therapy with auto-SCT (Arm B), used as consolidation after three CHOP courses in case of CR or very good PR. A total of 86 patients were enrolled, of which 39 and 43 patients were evaluable in arm A and arm B, respectively. The primary endpoint was PFS. On an intent-to-treat basis and with a median follow-up time of 77.1 (range 1–135.5) months, the median PFS was 22 months in Arm A and 53 months in Arm B (P<0.0001). Median survival time was 104.7 months in arm A and 107.4 months in arm B. This trial demonstrates that frontline high-dose therapy with auto-SCT prolongs PFS but does not translate into a survival advantage in advanced CLL patients in the pre-rituximab era.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Mauro FR, Foa R, Giannarelli D, Cordone I, Crescenzi S, Pescarmona E et al. Clinical characteristics and outcome of young chronic lymphocytic leukemia patients: a single institution study of 204 cases. Blood 1999; 94: 448–454.
Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 1999; 94: 1840–1847.
Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK . Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999; 94: 1848–1854.
Dohner H, Stilgenbauer S, Benner A, Leupolt E, Kröber A, Bullinger L et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1910–1916.
Rai KR, Peterson BL, Appelbaum FR, Kolitz J, Elias L, Shepherd L et al. Fludarabine compared with chlorambucil as primary therapy for chronic lymphocytic leukemia. N Engl J Med 2000; 343: 1750–1757.
Keating MJ, O’Brien S, Albitar M, Lerner S, Plunkett W, Giles F et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005; 23: 4079–4088.
Byrd CJ, Peterson BL, Morrisson VA, Park K, Jacobson R, Hoke E et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003; 101: 6–14.
Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010; 376: 1164–1174.
Dreger P, Stilgenbauer S, Benner A, Ritgen M, Kröber A, Kneba M et al. The prognostic impact of autologous stem cell transplantation in patients with chronic lymphocytic leukemia: a risk-matched analysis based on the VH gene mutational status. Blood 2004; 103: 2850–2858.
Gribben JG, Zahrieh D, Stephans C, Bartlett-Pandite L, Alyea E, Fisher D et al. Autologous and allogeneic stem cell transplantations for poor-risk chronic lymphocytic leukemia. Blood 2005; 106: 4389–4396.
Michallet M, Dreger P, Sutton L, Brand R, Richards S, Van Os M et al. Autologous stem cell transplantation in chronic lymphocytic leukemia: results of an European intergroup randomized trial comparing autografting versus observation. Blood 2010; 117: 1516–1521.
Cheson BD, Benett JM, Grever M, Kay N, Keating MJ, O’Brien S et al. National Cancer Institute-sponsored working group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood 1996; 87: 4990–4997.
Binet JL, Auquier A, Dighiero G, Chastang C, Piguet H, Goasguen J et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate analysis. Cancer 1981; 48: 198–206.
Dreger P, Monserrat E . Autologous and allogeneic stem cell transplantation for chronic lymphocytic leukemia. Leukemia 2002; 16: 985–992.
Leporrier M, Chevret S, Cazin B, Boudjerra N, Feugier P, Desablens B et al. Randomized comparison of fludarabine, CAP, and CHOP in 938 untreated stage B and C chronic lymphocytic leukemia. Blood 2001; 98: 2319–2325.
Van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL et al. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombination in suspect lymphoproliferations: report of the biomed-2 concerted action BMH4-CT98-3936. Leukemia 2003; 17: 2257–2317.
Matutes E, Owusu-Ankomah K, Morilla R, Garcia Marco J, Houlihan A, Que TH et al. The immunological profile of B-cell disorders and proposal of a scoring system for the diagnosis of CLL. Leukemia 1994; 8: 1640–1645.
Catovsky D, Richards S, Matutes E, Oscier D, Dyer MJ, Bezares RF et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukemia (The LFR CLL4 Trial): a randomised controlled trial. Lancet 2007; 370: 230–239.
Knauf W, Lissichkov T, Aldaoud A, Liberati A, Loscertales J, Herbrecht R et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukaemia. J Clin Oncol 2009; 27: 4378–4384.
Hillmen P, Skotnicki AB, Robak T, Jaksic B, Dmoszynska A, Wu J et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukaemia. J Clin Oncol 2007; 25: 5616–5623.
Eichhorst BF, Busch R, Hopfinger G, Pasold R, Hensel M, Steinbrecher C et al. Fludarabine plus cyclophosphamide versus fludarabine alone in first-line therapy of younger patients with chronic lymphocytic leukemia. Blood 2006; 107: 885–891.
Flinn IW, Neuberg DS, Grever MR, Dewald GW, Bennett JM, Paietta EM et al. Phase III trial of fludarabine plus cyclophosphamide compared with fludarabine for patients with previously untreated chronic lymphocytic leukemia: US Intergroup Trial E2997. J Clin Oncol 2007; 25: 793–798.
Provan D, Brtlett-Pandite L, Zwicky C, Neuberg D, Maddocks A, Corradini P et al. Eradication of polymerase chain reaction-detectable chronic lymphocytic leukemia cells is associated with improved outcome after bone marrow transplantation. Blood 1996; 88: 2228–2235.
Milligan DW, Fernandes S, Dasgupta R, Davies SE, Matutes E, Fegan CD et al. Results of the MRC pilot study show autografting for younger patients with chronic lymphocytic leukemia is safe and achieves a high percentage of molecular responses. Blood 2005; 105: 397–404.
Jantunen E, Itälä M, Siitonen T, Kuittinen T, Heiskanen J, Koivunen E et al. Blood stem cell mobilization and collection in patients with chronic lymphocytic leukaemia: a nationwide analysis. Bone Marrow Transplant 2008; 41: 239–244.
Stone RM, Neuberg D, Soiffer R, Takvorian T, Whelan M, Rabinowe SN et al. Myelodysplastic syndrome as a late complication following autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 1994; 12: 2535–2542.
Friedberg JW, Neuberg D, Stone RM, Alyea E, Jallow H, LaCasce E et al. Outcome in patients with myelodysplastic syndrome after autologous bone marrow transplantation for non-Hodgkin's lymphoma. J Clin Oncol 1999; 17: 3128–3135.
Gilliland DG, Gribben JG . Evaluation of the risk of therapy-related MDS/AML after autologous stem cell transplantation. Biol Blood Marrow Transplant 2002; 14: 10–18.
Bosch F, Ferrer A, Lopez-Guillermo A, Gine E, Bellosillo B, Villamor N et al. Fludarabine, cyclophosphamide and mitoxantrone in the treatment of resistant or relapsed chronic lymphocytic leukaemia. Br J Haematol 2002; 119: 976–984.
Esteve J, Villamor N, Colomer D, Cervantes F, Campo E, Carreras E et al. Stem cell transplantation for chronic lymphocytic leukemia: different outcome after autologous and allogeneic transplantation and correlation with minimal residual disease status. Leukemia 2001; 15: 445–451.
Moreton P, Kennedy B, Lucas G, Leach M, Rassam SM, Haynes A et al. Eradication of minimal residual disease in B-Cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol 2005; 23: 2971–2979.
Montserrat E . Treatment of chronic lymphocytic leukaemia: achieving minimal residual disease-negative as a goal. J Clin Oncol 2005; 23: 2884–2885.
Dreger P, Ritgen M, Böttcher S, Schmitz N, Kneba M . The prognostic impact of minimal residual disease assessment after stem cell transplantation for chronic lymphocytic leukaemia: is achievement of molecular remission worthwhile? Leukemia 2005; 19: 1135–1138.
Tam CS, O’Brien S, WIerda W, Kantarjian H, Wen S, Do KA et al. Long-term results of the fludarabine, cyclophosphamide and rituximab regimen as initial therapy of chronic lymphocytic leukaemia. Blood 2008; 112: 975–980.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Brion, A., Mahé, B., Kolb, B. et al. Autologous transplantation in CLL patients with B and C Binet stages: final results of the prospective randomized GOELAMS LLC 98 trial. Bone Marrow Transplant 47, 542–548 (2012). https://doi.org/10.1038/bmt.2011.117
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.117
Keywords
This article is cited by
-
Secondary malignancies following high dose therapy and autologous hematopoietic cell transplantation-systematic review and meta-analysis
Bone Marrow Transplantation (2015)
-
High-dose therapy and autologous hematopoietic cell transplantation as front-line consolidation in chronic lymphocytic leukemia: a systematic review
Bone Marrow Transplantation (2015)
-
Results of a randomized trial comparing high-dose chemotherapy plus Auto-SCT and R-FC in CLL at diagnosis
Bone Marrow Transplantation (2014)