Abstract
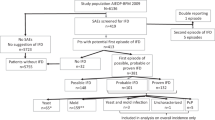
Patients on systemic glucocorticoids for GVHD after hematopoietic cell transplant are susceptible to invasive fungal infections (IFI), which greatly contribute to morbidity and mortality. We evaluated the efficacy of prophylactic treatment options (voriconazole or fluconazole vs itraconazole) for IFI by performing a retrospective review of patients on glucocorticoids for GVHD who were administered voriconazole (n=97), fluconazole (n=36) or itraconazole (n=36). IFI developed in 7/72 (10%) patients on fluconazole/itraconazole vs 2/97 (2%) on voriconazole (P=0.03) within the first 100 days of glucocorticoids. Five (7%) patients developed Aspergillus IFI on fluconazole/itraconazole, compared with none on voriconazole (0%) (P=0.008); Aspergillus IFI resulted in death in all five patients. We found that IFI occurred in patients who received an initial dose of at least 2 mg/kg/day of prednisone or equivalent; when the analysis was restricted to these patients, the hazard ratio (0.39; 95% confidence interval: 0.08–1.86) was consistent with a protective effect of voriconazole compared with fluconazole/itraconazole, although this subset analysis did not reach significance. OS at 100 days after start of glucocorticoids was 77% in patients administered fluconazole/itraconazole and 85% in those administered voriconazole (P=0.22). Our results suggest that voriconazole is more effective than fluconazole/itraconazole in preventing IFI, especially aspergillosis, in patients receiving glucocorticoids post transplant.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Wingard JR . Fungal infections after bone marrow transplant. Biol Blood Marrow Transplant 1999; 5: 55–68.
Wald A, Leisenring W, van Burik J-A, Bowden RA . Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 1997; 175: 1459–1466.
Chamilos G, Luna M, Lewis RE, Bodey GP, Chemaly R, Tarrand JJ et al. Invasive fungal infections in patients with hematologic malignancies in a tertiary care cancer center: an autopsy study over a 15-year period (1989–2003). Haematologica 2006; 91: 986–989.
Hagen EA, Stern H, Porter D, Duffy K, Foley K, Luger S et al. High rate of invasive fungal infections following nonmyeloablative allogeneic transplantation. Clin Infect Dis 2003; 36: 9–15.
Parody R, Martino R, Rovira M, Vazquez L, Vázquez MJ, de la Cámara R et al. Severe infections after unrelated donor allogeneic hematopoietic stem cell transplantation in adults: comparison of cord blood transplantation with peripheral blood and bone marrow transplantation. Biol Blood Marrow Transplant 2006; 12: 734–748.
Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Eng J Med 1992; 326: 845–851.
Rotstein C, Bow EJ, Laverdiere M, Ioannou S, Carr D, Moghaddam N . Randomized placebo-controlled trial of fluconazole prophylaxis for neutropenic cancer patients: benefit based on purpose and intensity of cytotoxic therapy. The Canadian Fluconazole Prophylaxis Study Group. Clin Infect Dis 1999; 28: 331–340.
Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis 1995; 171: 1545–1552.
Marr KA, Seidel K, Slavin M, Bowden RA, Schoch HG, Flowers ME et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized, placebo-controlled trial. Blood 2000; 96: 2055–2061.
Winston DJ, Maziarz RT, Chandrasekar PH, Lazarus HM, Goldman M, Blumer JL et al. Intravenous and oral itraconazole versus intravenous and oral fluconazole for long-term antifungal prophylaxis in allogeneic hematopoietic stem-cell transplant recipients. A multicenter, randomized trial. Ann Intern Med 2003; 138: 705–713.
Marr KA, Crippa F, Leisenring W, Hoyle M, Boeckh M, Balajee SA et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood 2004; 103: 1527–1533.
Ullmann AJ, Lipton JH, Vesole DH, Chandrasekar P, Langston A, Tarantolo SR et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med 2007; 356: 335–347.
Marco F, Pfaller MA, Messer S, Jones RN . In vitro activities of voriconazole (UK-109, 496) and four other antifungal agents against 394 clinical isolates of Candida sp. Antimicrob Agents Chemother 1998; 42: 161–163.
Purkins L, Wood N, Ghahramani P, Greenleigh K, Allen MJ, Kleinermans D . Pharmacokinetics and safety of voriconazole following intravenous to oral-dose escalation regimens. Antimicrob Agents Chemother 2002; 46: 2546–2553.
Lazarus HM, Blumer JL, Yanovich S, Schlamm H, Romero A . Safety and pharmacokinetics of oral voriconazole in patients at risk of fungal infection: a dose escalation study. J Clin Pharmacol 2002; 42: 395–402.
Ascioglu S, Rex JH, de Pauw B, Bennett JE, Bille J, Crokaert F et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis 2002; 34: 7–14.
Marubini E, Valsecchi MG . Analyzing Survival Data from Clinical Trials and Observational Studies. John Wiley & Sons Ltd: Chichester, UK, 1995.
Gray RJ . A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988; 16: 1140–1154.
Marr KA, Carter RA, Boeckh M, Martin P, Corey L . Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 2002; 100: 4358–4366.
Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplantation after nonmyeloablative conditioning. Blood 2003; 102: 823–833.
Garcia-Vidal C, Upton A, Kirby KA, Marr KA . Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 2008; 47: 1041–1050.
Wingard JR, Carter SL, Walsh TJ, Kurtzberg J, Small TN, Gersten ID et al. Results of a randomized, double-blind trial of fluconazole vs voriconazole for the prevention of invasive fungal infections in 600 allogeneic blood and marrow transplant patients. Blood 2007; 110: 163 (abstract).
Acknowledgements
JP and UG were supported by Pfizer and CA was supported by the National Cancer Institute (Grant 3 P30-CA7692-09).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gergis, U., Markey, K., Greene, J. et al. Voriconazole provides effective prophylaxis for invasive fungal infection in patients receiving glucocorticoid therapy for GVHD. Bone Marrow Transplant 45, 662–667 (2010). https://doi.org/10.1038/bmt.2009.210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2009.210
Keywords
This article is cited by
-
Impact of oral voriconazole during chemotherapy for acute myeloid leukemia and myelodysplastic syndrome: a Japanese nationwide retrospective cohort study
International Journal of Clinical Oncology (2019)
-
Foiling fungal disease post hematopoietic cell transplant: review of prophylactic strategies
Bone Marrow Transplantation (2018)
-
‘To treat or not to treat’: raising awareness on the effects of graft versus host disease drugs on musculoskeletal system
Bone Marrow Transplantation (2018)
-
Invasive Candidiasis in the Neutropenic Cancer Patient
Current Fungal Infection Reports (2011)
-
High-Risk Myelodysplastic Syndromes: Chemotherapy, Transplantation, and Beyond
Current Hematologic Malignancy Reports (2010)