Abstract
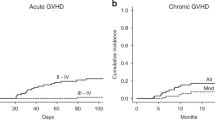
We developed a novel algorithm to define the need for high-dose prophylactic i.v. Igs (IVIG) in periods of high risk for CMV to patients after allo-SCT. IVIG were administered only if at least one of the following, monthly-assessed, criteria was fulfilled: (1) IgG concentration <4 g/l, (2) NK (natural killer) cell count <100/μl, (3) CD4+ cell count <100/μl, (4) acute or chronic GVHD. The primary endpoint was to determine the cumulative incidence of CMV infection in patients who received prophylactic IVIG according to the algorithm (intervention group) and compare it with that of a sequentially assessed control group, to which prophylactic IVIG were not administered. The study included 79 patients (44 in the intervention and 35 in the control group). The estimated cumulative incidence of CMV infection in the intervention and control group did not differ significantly (44 and 36%; P=0.31). Additionally, prophylactic IVIG did not reduce the frequency of CMV infection episodes. CMV disease was rare in both cohorts (5 and 9%; P=0.65). We conclude that prophylactic IVIG should not be administered after allo-SCT, even if administered selectively in a high dose to patients with delayed immune reconstitution or GVHD.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101: 407–414.
Bjorklund A, Aschan J, Labopin M, Remberger M, Ringden O, Winiarski J et al. Risk factors for fatal infectious complications developing late after allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 40: 1055–1062.
Ozdemir E, Saliba RM, Champlin RE, Couriel DR, Giralt SA, de Lima M et al. Risk factors associated with late cytomegalovirus reactivation after allogeneic stem cell transplantation for hematological malignancies. Bone Marrow Transplant 2007; 40: 125–136.
Einsele H, Hebart H, Kauffmann-Schneider C, Sinzger C, Jahn G, Bader P et al. Risk factors for treatment failures in patients receiving PCR-based preemptive therapy for CMV infection. Bone Marrow Transplant 2000; 25: 757–763.
Ljungman P, Brand R, Einsele H, Frassoni F, Niederwieser D, Cordonnier C . Donor CMV serologic status and outcome of CMV-seropositive recipients after unrelated donor stem cell transplantation: an EBMT megafile analysis. Blood 2003; 102: 4255–4260.
Boeckh M, Nichols WG . The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004; 103: 2003–2008.
Chakrabarti S, Milligan DW, Brown J, Osman H, Vipond IB, Pamphilon DH et al. Influence of cytomegalovirus (CMV) sero-positivity on CMV infection, lymphocyte recovery and non-CMV infections following T-cell-depleted allogeneic stem cell transplantation: a comparison between two T-cell depletion regimens. Bone Marrow Transplant 2004; 33: 197–204.
Hambach L, Stadler M, Dammann E, Ganser A, Hertenstein B . Increased risk of complicated CMV infection with the use of mycophenolate mofetil in allogeneic stem cell transplantation. Bone Marrow Transplant 2002; 29: 903–906.
Ganepola S, Gentilini C, Hilbers U, Lange T, Rieger K, Hofmann J et al. Patients at high risk for CMV infection and disease show delayed CD8+ T-cell immune recovery after allogeneic stem cell transplantation. Bone Marrow Transplant 2007; 39: 293–299.
Scholl S, Mügge LO, Issa MC, Kasper C, Pachmann K, Höffken K et al. Impact of early NK cell recovery on development of GVHD and CMV reactivation in dose-reduced regimen prior to allogeneic PBSCT. Bone Marrow Transplant 2005; 35: 183–190.
Andrei G, De Clercq E, Snoeck R . Novel inhibitors of human CMV. Curr Opin Investig Drugs 2008; 9: 132–145.
Einsele H, Kapp M, Grigoleit GU . CMV-specific T cell therapy. Blood Cells Mol Dis 2008; 40: 71–75.
Maury S, Mary JY, Rabian C, Schwarzinger M, Toubert A, Scieux C et al. Prolonged immune deficiency following allogeneic stem cell transplantation: risk factors and complications in adult patients. Br J Haematol 2001; 115: 630–641.
Cordonnier C, Chevret S, Legrand M, Rafi H, Dhédin N, Lehmann B, et al., GREFIG Study Group. Should immunoglobulin therapy be used in allogeneic stem-cell transplantation? A randomized, double-blind, dose effect, placebo-controlled, multicenter trial. Ann Intern Med 2003; 139: 8–18.
Winston DJ, Ho WG, Bartoni K, Champlin RE . Intravenous immunoglobulin and CMV-seronegative blood products for prevention of CMV infection and disease in bone marrow transplant recipients. Bone Marrow Transplant 1993; 12: 283–288.
Sullivan KM, Kopecky KJ, Jocom J, Fisher L, Buckner CD, Meyers JD et al. Immunomodulatory and antimicrobial efficacy of intravenous immunoglobulin in bone marrow transplantation. N Engl J Med 1990; 323: 705–712.
Tha-In T, Metselaar HJ, Tilanus HW, Boor PP, Mancham S, Kuipers EJ et al. Superior immunomodulatory effects of intravenous immunoglobulins on human T-cells and dendritic cells: comparison to calcineurin inhibitors. Transplantation 2006; 81: 1725–1734.
Winston DJ, Ho WG, Lin CH, Bartoni K, Budinger MD, Gale RP et al. Intravenous immune globulin for prevention of cytomegalovirus infection and interstitial pneumonia after bone marrow transplantation. Ann Intern Med 1987; 106: 12–18.
Centers for Disease Control and Prevention, Infectious Disease Society of America, American Society of Blood and Marrow Transplantation. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep 2000; 49: 1–125 CE1–7.
Wolff SN, Fay JW, Herzig RH, Greer JP, Dummer S, Brown RA et al. High-dose weekly intravenous immunoglobulin to prevent infections in patients undergoing autologous bone marrow transplantation or severe myelosuppressive therapy. A study of the American Bone Marrow Transplant Group. Ann Intern Med 1993; 118: 937–942.
Sullivan KM, Storek J, Kopecky KJ, Jocom J, Longton G, Flowers M et al. A controlled trial of long-term administration of intravenous immunoglobulin to prevent late infection and chronic graft-vs-host disease after marrow transplantation: clinical outcome and effect on subsequent immune recovery. Biol Blood Marrow Transplant 1996; 2: 44–53.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Lee SJ, Vogelsang G, Flowers ME . Chronic graft-versus-host disease. Biol Blood Marrow Transplant 2003; 9: 215–233.
Yakushiji K, Gondo H, Kamezaki K, Shigematsu K, Hayashi S, Kuroiwa M et al. Monitoring of cytomegalovirus reactivation after allogeneic stem cell transplantation: comparison of an antigenemia assay and quantitative real-time polymerase chain reaction. Bone Marrow Transplant 2002; 29: 599–606.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Akhan H, Cordonnier C . Antimicrobial prophylaxis in EBMT centers: a report from the EBMT Infectious Diseases Working Party (Abstract). Bone Marrow Transplant 2001; 27 (Suppl 1): S202.
Bass EB, Powe NR, Goodman SN, Graziano SL, Griffiths RI, Kickler TS et al. Efficacy of immune globulin in preventing complications of bone marrow transplantation: a meta-analysis. Bone Marrow Transplant 1993; 12: 273–282.
Guglielmo BJ, Wong-Beringer A, Linker CA . Immune globulin therapy in allogeneic bone marrow transplant: a critical review. Bone Marrow Transplant 1994; 13: 499–510.
Feinstein LC, Seidel K, Jocum J, Bowden RA, Anasetti C, Deeg HJ et al. Reduced dose intravenous immunoglobulin does not decrease transplant-related complications in adults given related donor marrow allografts. Biol Blood Marrow Transplant 1999; 5: 369–378.
Norlin AC, Sairafi D, Mattsson J, Ljungman P, Ringdén O, Remberger M . Allogeneic stem cell transplantation: low immunoglobulin levels associated with decreased survival. Bone Marrow Transplant 2008; 41: 267–273.
Acknowledgements
We thank Mrs C Grobecker for the thorough data documentation. We also thank Grifols GmbH, Langen, Germany for financially supporting the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schmidt-Hieber, M., Schwarck, S., Stroux, A. et al. Prophylactic i.v. Igs in patients with a high risk for CMV after allo-SCT. Bone Marrow Transplant 44, 185–192 (2009). https://doi.org/10.1038/bmt.2008.435
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.435
Keywords
This article is cited by
-
Infectious diseases in allogeneic haematopoietic stem cell transplantation: prevention and prophylaxis strategy guidelines 2016
Annals of Hematology (2016)
-
The prevention and treatment of cytomegalovirus infection in haematopoietic stem cell transplantation
Cancer Immunology, Immunotherapy (2009)