Abstract
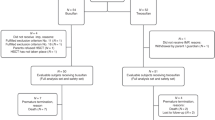
To evaluate the toxicity and efficacy of an i.v. preparation of BU (12.8 mg/kg), combined with CY (120 mg/kg), a prospective study was performed on 30 Japanese patients (median age, 30 years) with hematologic malignancies undergoing hematopoietic SCT (28 allogeneic transplants from an HLA-matched donor and 2 autologous transplants). There were no significant toxicities, and all but one patient showed evidence of granulocyte engraftment at a median of 14 days for allogeneic and 11 days for autologous transplantation. Grades II–IV acute and chronic GVHD occurred in 9 (9/27, 33%) and 16 patients (16/27, 59%), respectively. Non-relapse mortality at days 100 and 365 was 3 and 17%, respectively. The pharmacokinetics of i.v. BU showed close inter- and intrapatient consistency; the area under the plasma concentration–time curve of the first administration remained at less than 1500 μmol min/l in 27 of the 29 patients (93%), and between 900 and 1350 μmol min/l in 22 patients (73%). As all of the profiles overlap with data from non-Japanese patients, we conclude that racial factors may not seriously influence the bioactivity of i.v. BU.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Litzow MR, Pérez WS, Klein JP, Bolwell BJ, Camitta B, Copelan EA et al. Comparison of outcome following allogeneic bone marrow transplantation with cyclophosphamide–total body irradiation versus busulphan–cyclophosphamide conditioning regimens for acute myelogenous leukaemia in first remission. Br J Haematol 2002; 119: 1115–1124.
Vassal G, Deroussent A, Hartmann O, Challine D, Benhamou E, Valteau-Couanet D et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res 1990; 50: 6203–6207.
Hassan M, Ljungman P, Bolme P, Ringdn O, Syrckov Z, Bekassy A et al. Busulfan bioavailability. Blood 1994; 84: 2144–2150.
Schuler U, Schroer S, Kühnle A, Blanz J, Mewes K, Kumbier I et al. Busulfan pharmacokinetics in bone marrow transplant patients: is drug monitoring warranted? Bone Marrow Transplant 1994; 14: 759–765.
Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen TL, Saral R et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55–61.
Hassan M, Oberg G, Ehrsson H, Ehrnebo M, Wallin I, Smedmyr B et al. Pharmacokinetic and metabolic studies of high-dose busulphan in adults. Eur J Clin Pharmacol 1989; 36: 525–530.
Grochow LB . Busulfan disposition: the role of therapeutic monitoring in bone marrow transplantation induction regimens. Semin Oncol 1993; 20 (Suppl. 4): 18–25.
Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225–230.
Copelan EA, Bechtel TP, Avalos BR, Elder PJ, Ezzone SA, Scholl MD et al. Busulfan levels are influenced by prior treatment and are associated with hepatic veno-occlusive disease and early mortality but not with delayed complications following marrow transplantation. Bone Marrow Transplant 2001; 27: 1121–1124.
Vassal G, Re M, Gouyette A . Gas chromatographic-mass spectrometric assay for busulfan in biological fluids using a deuterated internal standard. J Chromatogr 1988; 428: 357–361.
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI et al. Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 1997; 89: 3055–3060.
McCune JS, Gooley T, Gibbs JP, Sanders JE, Petersdorf EW, Appelbaum FR et al. Busulfan concentration and graft rejection in pediatric patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2002; 30: 167–173.
Bolinger AM, Zangwill AB, Slattery JT, Glidden D, DeSantes K, Heyn L et al. An evaluation of engraftment, toxicity and busulfan concentration in children receiving bone marrow transplantation for leukemia or genetic disease. Bone Marrow Transplant 2000; 25: 925–930.
Bolinger AM, Zangwill AB, Slattery JT, Risler LJ, Sultan DH, Glidden DV et al. Target dose adjustment of busulfan in pediatric patients undergoing bone marrow transplantation. Bone Marrow Transplant 2001; 28: 1013–1018.
Deeg HJ, Storer B, Slattery JT, Anasetti C, Doney KC, Hansen JA et al. Conditioning with targeted busulfan and cyclophosphamide for hemopoietic stem cell transplantation from related and unrelated donors in patients with myelodysplastic syndrome. Blood 2002; 100: 1201–1207.
Kami M, Hamaki T, Maruta Y, Miyakoshi S, Mutou Y . Limitations of oral busulfan in preparative regimen before hematopoietic stem-cell transplantation. Haematologica 2002; 87: ELT10.
Andersson BS, Madden T, Tran HT, Hu WW, Blume KG, Chow DS et al. Acute safety and pharmacokinetics of intravenous busulfan when used with oral busulfan and cyclophosphamide as pretransplantation conditioning therapy: a phase I study. Biol Blood Marrow Transplant 2000; 6: 548–554.
Andersson BS, Gajewski J, Donato M, Giralt S, Gian V, Wingard J et al. Allogeneic stem cell transplantation (BMT) for AML and MDS following i.v. busulfan and cyclophosphamide (i.v. BuCy). Bone Marrow Transplant 2000; 25 (Suppl 2): S35–S38.
Andersson BS, Kashyap A, Gian V, Wingard JR, Fernandez H, Cagnoni PJ et al. Conditioning therapy with intravenous busulfan and cyclophosphamide (IV BuCy2) for hematologic malignancies prior to allogeneic stem cell transplantation: a phase II study. Biol Blood Marrow Transplant 2002; 8: 145–154.
Shimoni A, Bielorai B, Toren A, Hardan I, Avigdor A, Yeshurun M et al. Intravenous busulfan-based conditioning prior to allogeneic hematopoietic stem cell transplantation: myeloablation with reduced toxicity. Exp Hematol 2003; 31: 428–434.
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 2002; 8: 493–500.
Takama H, Tanaka H, Nakashima D, Ueda R, Takaue Y . Population pharmacokinetics of intravenous busulfan in patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant 2006; 37: 345–351.
Kaplan El, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED . Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116–122.
Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation 1987; 44: 778–783.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant 1995; 15: 825–828.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med 1980; 69: 204–217.
Xie HG, Kim RB, Wood AJ, Stein CM . Molecular basis of ethnic differences in drug disposition and response. Annu Rev Pharmacol Toxicol 2001; 41: 815–850.
Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347–1353.
Nguyen L, Fuller D, Lennon S, Leger F, Puozzo C . I.V. busulfan in pediatrics: a novel dosing to improve safety/efficacy for hematopoietic progenitor cell transplantation recipients. Bone Marrow Transplant 2004; 33: 979–987.
Acknowledgements
We thank Kirin Pharma Co. Ltd. (Japan) and PDL Bio Pharma Inc. (USA) for their support for this study. This study was conducted as a pivotal trial supported by Kirin Pharma Co. Ltd., Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, SW., Mori, Si., Tanosaki, R. et al. Busulfex (i.v. BU) and CY regimen before SCT: Japanese-targeted phase II pharmacokinetics combined study. Bone Marrow Transplant 43, 611–617 (2009). https://doi.org/10.1038/bmt.2008.372
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.372
Keywords
This article is cited by
-
Reduced-intensity conditioning is effective for hematopoietic stem cell transplantation in young pediatric patients with Diamond–Blackfan anemia
Bone Marrow Transplantation (2021)
-
Reduced-intensity stem cell transplantation for acute myeloid leukemia with fludarabine-based conditioning with intravenous busulfan versus melphalan
Bone Marrow Transplantation (2020)
-
Comparison of levetiracetam with phenytoin for the prevention of intravenous busulfan-induced seizures in hematopoietic cell transplantation recipients
Cancer Chemotherapy and Pharmacology (2018)
-
Pharmacokinetics study of once-daily intravenous busulfan in conditioning regimens for hematopoietic stem cell transplantation
International Journal of Hematology (2015)