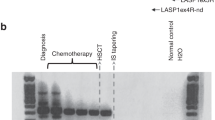
Abstract
Allogeneic SCT is important in myelodysplastic syndrome, the BCR-ABL-negative chronic myeloproliferative diseases (CMPDs) and in poor-risk AML. Techniques to monitor the minimal residual disease, for example, by PCR or immunophenotyping gain increasing importance in the post transplantation period as basis for improved and earlier therapeutic interventions in impending relapse. Recent markers such as the NPM1 mutations in AML or the JAK2V617F mutation in the CMPD can be exactly quantified by real-time PCR and were evaluated for their prognostic value in the post transplantation phase and for their utility to plan adoptive immunotherapy in case of molecular relapse. With respect to chimerism, new and very sensitive methods were introduced, for example, quantitative assessment of genetic polymorphisms by real-time PCR, but also methods here are still highly individualized. Only in CML, where SCT focuses now on poor-risk cases or cases of tyrosine kinase inhibitor failure, follow-up schedules are standardized. Standardization of the different diagnostic techniques and of the intervals in the post transplantation period is urgently needed also in other myeloid malignancies and should be focus of future studies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Bloomfield CD, Shuma C, Regal L, Philip PP, Hossfeld DK, Hagemeijer AM et al. Long-term survival of patients with acute myeloid leukemia: a third follow-up of the Fourth International Workshop on Chromosomes in Leukemia. Cancer 1997; 80: 2191–2198.
Marcucci G, Mrozek K, Bloomfield CD . Molecular heterogeneity and prognostic biomarkers in adults with acute myeloid leukemia and normal cytogenetics. Curr Opin Hematol 2005; 12: 68–75.
Swansbury GJ, Lawler SD, Alimena G, Arthur D, Berger R, Van den BH et al. Long-term survival in acute myelogenous leukemia: a second follow-up of the Fourth International Workshop on Chromosomes in Leukemia. Cancer Genet Cytogenet 1994; 73: 1–7.
Gratwohl A, Baldomero H, Frauendorfer K, Urbano-Ispizua A, Niederwieser D . Results of the EBMT activity survey 2005 on haematopoietic stem cell transplantation: focus on increasing use of unrelated donors. Bone Marrow Transplant 2007; 39: 71–87.
Storb R . Can reduced-intensity allogeneic transplantation cure older adults with AML? Best Pract Res Clin Haematol 2007; 20: 85–90.
Kroger N, Badbaran A, Holler E, Hahn J, Kobbe G, Bornhauser M et al. Monitoring of the JAK2-V617F mutation by highly sensitive quantitative real-time PCR after allogeneic stem cell transplantation in patients with myelofibrosis. Blood 2007; 109: 1316–1321.
Schmid C, Schleuning M, Schwerdtfeger R, Hertenstein B, Mischak-Weissinger E, Bunjes D et al. Long-term survival in refractory acute myeloid leukemia after sequential treatment with chemotherapy and reduced-intensity conditioning for allogeneic stem cell transplantation. Blood 2006; 108: 1092–1099.
Baccarani M, Saglio G, Goldman J, Hochhaus A, Simonsson B, Appelbaum F et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood 2006; 108: 1809–1820.
Hughes T, Deininger M, Hochhaus A, Branford S, Radich J, Kaeda J et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood 2006; 108: 28–37.
Kolb HJ, Schattenberg A, Goldman JM, Hertenstein B, Jacobsen N, Arcese W et al. Graft-versus-leukemia effect of donor lymphocyte transfusions in marrow grafted patients. Blood 1995; 86: 2041–2050.
Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E et al. Durability of responses following donor lymphocyte infusions for patients who relapse after allogeneic stem cell transplantation for chronic myeloid leukemia. Blood 2000; 96: 2712–2716.
Bornhauser M, Kroger N, Schwerdtfeger R, Schafer-Eckart K, Sayer HG, Scheid C et al. Allogeneic haematopoietic cell transplantation for chronic myelogenous leukaemia in the era of imatinib: a retrospective multicentre study. Eur J Haematol 2006; 76: 9–17.
Giralt SA, Arora M, Goldman JM, Lee SJ, Maziarz RT, McCarthy PL et al. Impact of imatinib therapy on the use of allogeneic haematopoietic progenitor cell transplantation for the treatment of chronic myeloid leukaemia. Br J Haematol 2007; 137: 461–467.
Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1031–1037.
O'Brien SG, Guilhot F, Larson RA, Gathmann I, Baccarani M, Cervantes F et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Jabbour E, Kantarjian HM, Abruzzo LV, O'Brien S, Garcia-Manero G, Verstovsek S et al. Chromosomal abnormalities in Philadelphia chromosome negative metaphases appearing during imatinib mesylate therapy in patients with newly diagnosed chronic myeloid leukemia in chronic phase. Blood 2007; 110: 2991–2995.
Kovitz C, Kantarjian H, Garcia-Manero G, Abruzzo LV, Cortes J . Myelodysplastic syndromes and acute leukemia developing after imatinib mesylate therapy for chronic myeloid leukemia. Blood 2006; 108: 2811–2813.
Grimwade D, Howe K, Langabeer S, Burnett A, Goldstone A, Solomon E . Minimal residual disease detection in acute promyelocytic leukemia by reverse-transcriptase PCR: evaluation of PML-RAR alpha and RAR alpha-PML assessment in patients who ultimately relapse. Leukemia 1996; 10: 61–66.
Leroy H, de BS, Grardel-Duflos N, Darre S, Leleu X, Roumier C et al. Prognostic value of real-time quantitative PCR (RQ-PCR) in AML with t(8;21). Leukemia 2005; 19: 367–372.
Schnittger S, Weisser M, Schoch C, Hiddemann W, Haferlach T, Kern W . Score predicting for prognosis in PML-RARA+, AML1-ETO+, or CBFBMYH11+ acute myeloid leukemia based on quantification of fusion transcripts. Blood 2003; 102: 2746–2755.
Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med 2005; 352: 254–266.
Schnittger S, Schoch C, Kern W, Mecucci C, Tschulik C, Martelli MF et al. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005; 106: 3733–3739.
Thiede C, Koch S, Creutzig E, Steudel C, Illmer T, Schaich M et al. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006; 107: 4011–4020.
James C, Ugo V, Casadevall N, Constantinescu SN, Vainchenker W . A JAK2 mutation in myeloproliferative disorders: pathogenesis and therapeutic and scientific prospects. Trends Mol Med 2005; 11: 546–554.
Jelinek J, Oki Y, Gharibyan V, Bueso-Ramos C, Prchal JT, Verstovsek S et al. JAK2 mutation 1849G>T is rare in acute leukemias but can be found in CMML, Philadelphia chromosome-negative CML, and megakaryocytic leukemia. Blood 2005; 106: 3370–3373.
Levine RL, Loriaux M, Huntly BJ, Loh ML, Beran M, Stoffregen E et al. The JAK2V617F activating mutation occurs in chronic myelomonocytic leukemia and acute myeloid leukemia, but not in acute lymphoblastic leukemia or chronic lymphocytic leukemia. Blood 2005; 106: 3377–3379.
Tefferi A, Lasho TL, Gilliland G . JAK2 mutations in myeloproliferative disorders. N Engl J Med 2005; 353: 1416–1417.
Bacher U, Badbaran A, Fehse B, Zabelina T, Zander A, Kroger N . Quantitative monitoring of NPM1 mutations provides a valid minimal residual disease parameter following allogeneic stem cell transplantation (Submitted for publication).
Fehse B, Chukhlovin A, Kuhlcke K, Marinetz O, Vorwig O, Renges H et al. Real-time quantitative Y chromosome-specific PCR (QYCS-PCR) for monitoring hematopoietic chimerism after sex-mismatched allogeneic stem cell transplantation. J Hematother Stem Cell Res 2001; 10: 419–425.
Thiede C, Bornhauser M, Ehninger G . Strategies and clinical implications of chimerism diagnostics after allogeneic hematopoietic stem cell transplantation. Acta Haematol 2004; 112: 16–23.
Alizadeh M, Bernard M, Danic B, Dauriac C, Birebent B, Lapart C et al. Quantitative assessment of hematopoietic chimerism after bone marrow transplantation by real-time quantitative polymerase chain reaction. Blood 2002; 99: 4618–4625.
Guglielmi C, Arcese W, Dazzi F, Brand R, Bunjes D, Verdonck LF et al. Donor lymphocyte infusion for relapsed chronic myelogenous leukemia: prognostic relevance of the initial cell dose. Blood 2002; 100: 397–405.
Faderl S, Hochhaus A, Hughes T . Monitoring of minimal residual disease in chronic myeloid leukemia. Hematol Oncol Clin North Am 2004; 18: 657–670.
Apperley JF . Managing the patient with chronic myeloid leukemia through and after allogeneic stem cell transplantation. Hematology Am Soc Hematol Educ Program 2006, 226–232.
van Marion AM, Thiele J, Kvasnicka HM, van den Tweel JG . Morphology of the bone marrow after stem cell transplantation. Histopathology 2006; 48: 329–342.
Dirnhofer S, Went P, Tichelli A . Diagnostic problems in follow-up bone marrow biopsies of patients treated for acute and chronic leukaemias and MDS. Pathobiology 2007; 74: 115–120.
Lioznov M, Ikogho R, Fehse B, Bacher U, Kroger N et al. Factors predicting haematological reconstitution following haemopoietic stem cell transplantation. Bone Marrow Transplant (in press).
Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children's Leukaemia Working Parties. Blood 1998; 92: 2322–2333.
Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T . Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia 2004; 18: 120–125.
Fenaux P . Chromosome and molecular abnormalities in myelodysplastic syndromes. Int J Hematol 2001; 73: 429–437.
Sole F, Luno E, Sanzo C, Espinet B, Sanz GF, Cervera J et al. Identification of novel cytogenetic markers with prognostic significance in a series of 968 patients with primary myelodysplastic syndromes. Haematologica 2005; 90: 1168–1178.
Bacher U, Haferlach T, Kern W, Hiddemann W, Schnittger S, Schoch C . Conventional cytogenetics of myeloproliferative diseases other than CML contribute valid information. Ann Hematol 2005; 84: 250–257.
Bench AJ, Cross NC, Huntly BJ, Nacheva EP, Green AR . Myeloproliferative disorders. Best Pract Res Clin Haematol 2001; 14: 531–551.
Tefferi A, Mesa RA, Schroeder G, Hanson CA, Li CY, Dewald GW . Cytogenetic findings and their clinical relevance in myelofibrosis with myeloid metaplasia. Br J Haematol 2001; 113: 763–771.
Bacher U, Kern W, Schoch C, Schnittger S, Hiddemann W, Haferlach T . Evaluation of complete disease remission in acute myeloid leukemia: a prospective study based on cytomorphology, interphase fluorescence in situ hybridization, and immunophenotyping during follow-up in patients with acute myeloid leukemia. Cancer 2006; 106: 839–847.
Fuehrer M, Gerusel-Bleck M, Konstantopoulos N, der-Goetze C, Walther JU . FISH analysis of native smears from bone marrow and blood for the monitoring of chimerism and clonal markers after stem cell transplantation in children. Int J Mol Med 2005; 15: 291–297.
Haferlach T, Bacher U, Kern W, Schnittger S, Haferlach C . Diagnostic pathways in acute leukemias: a proposal for a multimodal approach. Ann Hematol 2007; 86: 311–327.
Christiansen DH, Andersen MK, Pedersen-Bjergaard J . Mutations of AML1 are common in therapy-related myelodysplasia following therapy with alkylating agents and are significantly associated with deletion or loss of chromosome arm 7q and with subsequent leukemic transformation. Blood 2004; 104: 1474–1481.
Chen CY, Lin LI, Tang JL, Ko BS, Tsay W, Chou WC et al. RUNX1 gene mutation in primary myelodysplastic syndrome—the mutation can be detected early at diagnosis or acquired during disease progression and is associated with poor outcome. Br J Haematol 2007; 139: 405–414.
Shimoni A, Nagler A . Clinical implications of minimal residual disease monitoring for stem cell transplantation after reduced intensity and nonmyeloablative conditioning. Acta Haematol 2004; 112: 93–104.
Schlenk RF, Corbacioglu A, Krauter J, Bullinger L, Morgan M, Spaeth D et al. Gene mutations as predictive markers for younger adults with normal karyotype AML. ASH Annual Meeting Abstracts 2006; 108: 6a.
Scholl S, Krause C, Loncarevic IF, Muller R, Kunert C, Wedding U et al. Specific detection of Flt3 point mutations by highly sensitive real-time polymerase chain reaction in acute myeloid leukemia. J Lab Clin Med 2005; 145: 295–304.
Campana D . Determination of minimal residual disease in leukaemia patients. Br J Haematol 2003; 121: 823–838.
Kern W, Voskova D, Schoch C, Hiddemann W, Schnittger S, Haferlach T . Determination of relapse risk based on assessment of minimal residual disease during complete remission by multiparameter flow cytometry in unselected patients with acute myeloid leukemia. Blood 2004; 104: 3078–3085.
Griesinger F, Piro-Noack M, Kaib N, Falk M, Renziehausen A, Troff C et al. Leukaemia-associated immunophenotypes (LAIP) are observed in 90% of adult and childhood acute lymphoblastic leukaemia: detection in remission marrow predicts outcome. Br J Haematol 1999; 105: 241–255.
Kern W, Haferlach C, Haferlach T, Schnittger S . Monitoring of minimal residual disease in acute myeloid leukemia. Cancer 2008; 112: 4–16.
Lion T, Watzinger F . Chimerism analysis following nonmyeloablative stem cell transplantation. Methods Mol Med 2006; 125: 275–295.
Muller-Berat N, Lion T . Chimerism and transplant-related diagnostics. Leukemia 2006; 20: 1358–1360.
Bader P, Klingebiel T, Schaudt A, Theurer-Mainka U, Handgretinger R, Lang P et al. Prevention of relapse in pediatric patients with acute leukemias and MDS after allogeneic SCT by early immunotherapy initiated on the basis of increasing mixed chimerism: a single center experience of 12 children. Leukemia 1999; 13: 2079–2086.
Huisman C, de Weger RA, de Vries L, Tilanus MG, Verdonck LF . Chimerism analysis within 6 months of allogeneic stem cell transplantation predicts relapse in acute myeloid leukemia. Bone Marrow Transplant 2007; 39: 285–291.
Baron F, Sandmaier BM . Chimerism and outcomes after allogeneic hematopoietic cell transplantation following nonmyeloablative conditioning. Leukemia 2006; 20: 1690–1700.
Najfeld V, Burnett W, Vlachos A, Scigliano E, Isola L, Fruchtman S . Interphase FISH analysis of sex-mismatched BMT utilizing dual color XY probes. Bone Marrow Transplant 1997; 19: 829–834.
Lapointe C, Forest L, Lussier P, Busque L, Lagace F, Perreault C et al. Sequential analysis of early hematopoietic reconstitution following allogeneic bone marrow transplantation with fluorescence in situ hybridization (FISH). Bone Marrow Transplant 1996; 17: 1143–1148.
Buno I, Nava P, Simon A, Gonzalez-Rivera M, Jimenez JL, Balsalobre P et al. A comparison of fluorescent in situ hybridization and multiplex short tandem repeat polymerase chain reaction for quantifying chimerism after stem cell transplantation. Haematologica 2005; 90: 1373–1379.
Rothberg PG, Gamis AS, Baker D . Use of DNA polymorphisms to monitor engraftment after allogeneic bone marrow transplantation. Clin Lab Med 1997; 17: 109–118.
Thiede C, Bornhauser M, Oelschlagel U, Brendel C, Leo R, Daxberger H et al. Sequential monitoring of chimerism and detection of minimal residual disease after allogeneic blood stem cell transplantation (BSCT) using multiplex PCR amplification of short tandem repeat-markers. Leukemia 2001; 15: 293–302.
Thiede C . Diagnostic chimerism analysis after allogeneic stem cell transplantation: new methods and markers. Am J Pharmacogenomics 2004; 4: 177–187.
Elmaagacli AH . Real-time PCR for monitoring minimal residual disease and chimerism in patients after allogeneic transplantation. Int J Hematol 2002; 76 (Suppl 2): 204–205.
Bader P, Niethammer D, Willasch A, Kreyenberg H, Klingebiel T . How and when should we monitor chimerism after allogeneic stem cell transplantation? Bone Marrow Transplant 2005; 35: 107–119.
McCann SR, Crampe M, Molloy K, Lawler M . Hemopoietic chimerism following stem cell transplantation. Transfus Apher Sci 2005; 32: 55–61.
Kristt D, Stein J, Yaniv I, Klein T . Assessing quantitative chimerism longitudinally: technical considerations, clinical applications and routine feasibility. Bone Marrow Transplant 2007; 39: 255–268.
Matthes-Martin S, Lion T, Haas OA, Frommlet F, Daxberger H, Konig M et al. Lineage-specific chimaerism after stem cell transplantation in children following reduced intensity conditioning: potential predictive value of NK cell chimaerism for late graft rejection. Leukemia 2003; 17: 1934–1942.
Lion T . Detection of impending graft rejection and relapse by lineage-specific chimerism analysis. Methods Mol Med 2007; 134: 197–216.
Lion T, Daxberger H, Dubovsky J, Filipcik P, Fritsch G, Printz D et al. Analysis of chimerism within specific leukocyte subsets for detection of residual or recurrent leukemia in pediatric patients after allogeneic stem cell transplantation. Leukemia 2001; 15: 307–310.
Mohty M, Avinens O, Faucher C, Viens P, Blaise D, Eliaou JF . Predictive factors and impact of full donor T-cell chimerism after reduced intensity conditioning allogeneic stem cell transplantation. Haematologica 2007; 92: 1004–1006.
Thiede C, Lutterbeck K, Oelschlagel U, Kiehl M, Steudel C, Platzbecker U et al. Detection of relapse by sequential monitoring of chimerism in circulating CD34+ cells. Ann Hematol 2002; 81 (Suppl 2): S27–S28.
Prinz E, Keil F, Kalhs P, Mitterbauer M, Rabitsch W, Rosenmayr A et al. Successful immunotherapy in early relapse of acute myeloid leukemia after nonmyeloablative allogeneic stem cell transplantation. Ann Hematol 2003; 82: 295–298.
Preisler HD, Priore R, Azarnia N, Barcos M, Raza A, Rakowski I et al. Prediction of response of patients with acute nonlymphocytic leukaemia to remission induction therapy: use of clinical measurements. Br J Haematol 1986; 63: 625–636.
Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood 2003; 101: 64–70.
Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol 2003; 21: 4642–4649.
Marcucci G, Mrozek K, Ruppert AS, Archer KJ, Pettenati MJ, Heerema NA et al. Abnormal cytogenetics at date of morphologic complete remission predicts short overall and disease-free survival, and higher relapse rate in adult acute myeloid leukemia: results from cancer and leukemia group B study 8461. J Clin Oncol 2004; 22: 2410–2418.
El-Rifai W, Ruutu T, Elonen E, Volin L, Knuutila S . Prognostic value of metaphase-fluorescence in situ hybridization in follow-up of patients with acute myeloid leukemia in remission. Blood 1997; 89: 3330–3334.
Gallagher RE, Yeap BY, Bi W, Livak KJ, Beaubier N, Rao S et al. Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood 2003; 101: 2521–2528.
Marcucci G, Caligiuri MA, Dohner H, Archer KJ, Schlenk RF, Dohner K et al. Quantification of CBFbeta/MYH11 fusion transcript by real time RT-PCR in patients with inv(16) acute myeloid leukemia. Leukemia 2001; 15: 1072–1080.
Krauter J, Gorlich K, Ottmann O, Lubbert M, Dohner H, Heit W et al. Prognostic value of minimal residual disease quantification by real-time reverse transcriptase polymerase chain reaction in patients with core binding factor leukemias. J Clin Oncol 2003; 21: 4413–4422.
de LA, Pautas C, Thomas X, de BS, Bordessoule D, Tilly H et al. Allogeneic stem cell transplantation in second rather than first complete remission in selected patients with good-risk acute myeloid leukemia. Bone Marrow Transplant 2005; 35: 767–773.
Grimwade D, Jamal R, Goulden N, Kempski H, Mastrangelo S, Veys P . Salvage of patients with acute promyelocytic leukaemia with residual disease following ABMT performed in second CR using all-trans retinoic acid. Br J Haematol 1998; 103: 559–562.
Yanada M, Matsuo K, Emi N, Naoe T . Efficacy of allogeneic hematopoietic stem cell transplantation depends on cytogenetic risk for acute myeloid leukemia in first disease remission: a metaanalysis. Cancer 2005; 103: 1652–1658.
Elmaagacli AH, Beelen DW, Kroll M, Trzensky S, Stein C, Schaefer UW . Detection of CBFbeta/MYH11 fusion transcripts in patients with inv(16) acute myeloid leukemia after allogeneic bone marrow or peripheral blood progenitor cell transplantation. Bone Marrow Transplant 1998; 21: 159–166.
Miyamoto T, Nagafuji K, Akashi K, Harada M, Kyo T, Akashi T et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood 1996; 87: 4789–4796.
Kottaridis PD, Gale RE, Frew ME, Harrison G, Langabeer SE, Belton AA et al. The presence of a FLT3 internal tandem duplication in patients with acute myeloid leukemia (AML) adds important prognostic information to cytogenetic risk group and response to the first cycle of chemotherapy: analysis of 854 patients from the United Kingdom Medical Research Council AML 10 and 12 trials. Blood 2001; 98: 1752–1759.
Schnittger S, Schoch C, Dugas M, Kern W, Staib P, Wuchter C et al. Analysis of FLT3 length mutations in 1003 patients with acute myeloid leukemia: correlation to cytogenetics, FAB subtype, and prognosis in the AMLCG study and usefulness as a marker for the detection of minimal residual disease. Blood 2002; 100: 59–66.
Cloos J, Goemans BF, Hess CJ, van Oostveen JW, Waisfisz Q, Corthals S et al. Stability and prognostic influence of FLT3 mutations in paired initial and relapsed AML samples. Leukemia 2006; 20: 1217–1220.
Schnittger S, Schoch C, Kern W, Hiddemann W, Haferlach T . FLT3 length mutations as marker for follow-up studies in acute myeloid leukaemia. Acta Haematol 2004; 112: 68–78.
Elmaagacli AH . Molecular methods used for detection of minimal residual disease following hematopoietic stem cell transplantation in myeloid disorders. Methods Mol Med 2007; 134: 161–178.
Scholl S, Loncarevic IF, Krause C, Kunert C, Clement JH, Hoffken K . Minimal residual disease based on patient specific Flt3-ITD and -ITT mutations in acute myeloid leukemia. Leuk Res 2005; 29: 849–853.
van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ . Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia 2003; 17: 1013–1034.
Dohner K, Schlenk RF, Habdank M, Scholl C, Rucker FG, Corbacioglu A et al. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: interaction with other gene mutations. Blood 2005; 106: 3740–3746.
Verhaak RG, Goudswaard CS, van PW, Bijl MA, Sanders MA, Hugens W et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005; 106: 3747–3754.
Falini B, Bolli N, Shan J, Martelli MP, Liso A, Pucciarini A et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc+ AML. Blood 2006; 107: 4514–4523.
Gorello P, Cazzaniga G, Alberti F, Dell'Oro MG, Gottardi E, Specchia G et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006; 20: 1103–1108.
Chou WC, Tang JL, Wu SJ, Tsay W, Yao M, Huang SY et al. Clinical implications of minimal residual disease monitoring by quantitative polymerase chain reaction in acute myeloid leukemia patients bearing nucleophosmin (NPM1) mutations. Leukemia 2007; 21: 998–1004.
Weisser M, Kern W, Rauhut S, Schoch C, Hiddemann W, Haferlach T et al. Prognostic impact of RT-PCR-based quantification of WT1 gene expression during MRD monitoring of acute myeloid leukemia. Leukemia 2005; 19: 1416–1423.
Ogawa H, Tamaki H, Ikegame K, Soma T, Kawakami M, Tsuboi A et al. The usefulness of monitoring WT1 gene transcripts for the prediction and management of relapse following allogeneic stem cell transplantation in acute type leukemia. Blood 2003; 101: 1698–1704.
Coustan-Smith E, Ribeiro RC, Rubnitz JE, Razzouk BI, Pui CH, Pounds S et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol 2003; 123: 243–252.
Voskova D, Schoch C, Schnittger S, Hiddemann W, Haferlach T, Kern W . Stability of leukemia-associated aberrant immunophenotypes in patients with acute myeloid leukemia between diagnosis and relapse: comparison with cytomorphologic, cytogenetic, and molecular genetic findings. Cytometry B Clin Cytom 2004; 62: 25–38.
Ito S, Ishida Y, Murai K, Kuriya S . Flow cytometric analysis of aberrant antigen expression of blasts using CD45 blast gating for minimal residual disease in acute leukemia and high-risk myelodysplastic syndrome. Leuk Res 2001; 25: 205–211.
Nagler A, Condiotti R, Rabinowitz R, Schlesinger M, Nguyen M, Terstappen LW . Detection of minimal residual disease (MRD) after bone marrow transplantation (BMT) by multi-parameter flow cytometry (MPFC). Med Oncol 1999; 16: 177–187.
Wells DA, Sale GE, Shulman HM, Myerson D, Bryant EM, Gooley T et al. Multidimensional flow cytometry of marrow can differentiate leukemic from normal lymphoblasts and myeloblasts after chemotherapy and bone marrow transplantation. Am J Clin Pathol 1998; 110: 84–94.
Perez-Simon JA, Caballero D, ez-Campelo M, Lopez-Perez R, Mateos G, Canizo C et al. Chimerism and minimal residual disease monitoring after reduced intensity conditioning (RIC) allogeneic transplantation. Leukemia 2002; 16: 1423–1431.
List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 2005; 352: 549–557.
Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T . Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia 2005; 19: 1345–1349.
Shih LY, Huang CF, Wang PN, Wu JH, Lin TL, Dunn P et al. Acquisition of FLT3 or N-ras mutations is frequently associated with progression of myelodysplastic syndrome to acute myeloid leukemia. Leukemia 2004; 18: 466–475.
Bacher U, Haferlach T, Kern W, Haferlach C, Schnittger S . A comparative study of molecular mutations in 381 patients with myelodysplastic syndrome and in 4130 patients with acute myeloid leukemia. Haematologica 2007; 92: 744–752.
Bader P, Niemeyer C, Weber G, Coliva T, Rossi V, Kreyenberg H et al. WT1 gene expression: useful marker for minimal residual disease in childhood myelodysplastic syndromes and juvenile myelo-monocytic leukemia? Eur J Haematol 2004; 73: 25–28.
Tamaki H, Ogawa H, Ohyashiki K, Ohyashiki JH, Iwama H, Inoue K et al. The Wilms' tumor gene WT1 is a good marker for diagnosis of disease progression of myelodysplastic syndromes. Leukemia 1999; 13: 393–399.
Tamura K, Kanazawa T, Suzuki M, Koitabashi M, Ogawa C, Morikawa A . Successful rapid discontinuation of immunosuppressive therapy at molecular relapse after allogeneic bone marrow transplantation in a pediatric patient with myelodysplastic syndrome. Am J Hematol 2006; 81: 139–141.
Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood 2003; 102: 394–403.
Schoch C, Schnittger S, Bursch S, Gerstner D, Hochhaus A, Berger U et al. Comparison of chromosome banding analysis, interphase- and hypermetaphase-FISH, qualitative and quantitative PCR for diagnosis and for follow-up in chronic myeloid leukemia: a study on 350 cases. Leukemia 2002; 16: 53–59.
Hochhaus A, Lin F, Reiter A, Skladny H, Hehlmann R, Goldman JM et al. Quantitative molecular methods to monitor the response of CML patients to interferon-alpha. Bone Marrow Transplant 1996; 17 (Suppl 3): S41–S44.
Hochhaus A, Lin F, Reiter A, Skladny H, Mason PJ, van Rhee F et al. Quantification of residual disease in chronic myelogenous leukemia patients on interferon-alpha therapy by competitive polymerase chain reaction. Blood 1996; 87: 1549–1555.
Kurzrock R, Gutterman JU, Talpaz M . The molecular genetics of Philadelphia chromosome-positive leukemias. N Engl J Med 1988; 319: 990–998.
Melo JV . The molecular biology of chronic myeloid leukaemia. Leukemia 1996; 10: 751–756.
Neumann F, Herold C, Hildebrandt B, Kobbe G, Aivado M, Rong A et al. Quantitative real-time reverse-transcription polymerase chain reaction for diagnosis of BCR-ABL positive leukemias and molecular monitoring following allogeneic stem cell transplantation. Eur J Haematol 2003; 70: 1–10.
Kim MH, Stewart J, Devlin C, Kim YT, Boyd E, Connor M . The application of comparative genomic hybridization as an additional tool in the chromosome analysis of acute myeloid leukemia and myelodysplastic syndromes. Cancer Genet Cytogenet 2001; 126: 26–33.
Martinelli G, Iacobucci I, Soverini S, Cilloni D, Saglio G, Pane F et al. Monitoring minimal residual disease and controlling drug resistance in chronic myeloid leukaemia patients in treatment with imatinib as a guide to clinical management. Hematol Oncol 2006; 24: 196–204.
DeAngelo DJ, Hochberg EP, Alyea EP, Longtine J, Lee S, Galinsky I et al. Extended follow-up of patients treated with imatinib mesylate (Gleevec) for chronic myelogenous leukemia relapse after allogeneic transplantation: durable cytogenetic remission and conversion to complete donor chimerism without graft-versus-host disease. Clin Cancer Res 2004; 10: 5065–5071.
Weisser M, Tischer J, Schnittger S, Schoch C, Ledderose G, Kolb HJ . A comparison of donor lymphocyte infusions or imatinib mesylate for patients with chronic myelogenous leukemia who have relapsed after allogeneic stem cell transplantation. Haematologica 2006; 91: 663–666.
Lange T, Deininger M, Brand R, Hegenbart U, Al-Ali H, Krahl R et al. BCR-ABL transcripts are early predictors for hematological relapse in chronic myeloid leukemia after hematopoietic cell transplantation with reduced intensity conditioning. Leukemia 2004; 18: 1468–1475.
Asnafi V, Rubio MT, Delabesse E, Villar E, Davi F, Damaj G et al. Prediction of relapse by day 100 BCR-ABL quantification after allogeneic stem cell transplantation for chronic myeloid leukemia. Leukemia 2006; 20: 793–799.
Olavarria E, Craddock C, Dazzi F, Marin D, Marktel S, Apperley JF et al. Imatinib mesylate (STI571) in the treatment of relapse of chronic myeloid leukemia after allogeneic stem cell transplantation. Blood 2002; 99: 3861–3862.
Branford S, Rudzki Z, Walsh S, Grigg A, Arthur C, Taylor K et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood 2002; 99: 3472–3475.
Cortes JE, Talpaz M, Giles F, O'Brien S, Rios MB, Shan J et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood 2003; 101: 3794–3800.
Gruber FX, Lamark T, Anonli A, Sovershaev MA, Olsen M, Gedde-Dahl T et al. Selecting and deselecting imatinib-resistant clones: observations made by longitudinal, quantitative monitoring of mutated BCR-ABL. Leukemia 2005; 19: 2159–2165.
Cortes J, Jabbour E, Kantarjian H, Yin CC, Shan J, O'Brien S et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood 2007; 110: 4005–4011.
Ernst T, Erben P, Muller MC, Paschka P, Schenk T, Hoffmann J et al. Dynamics of BCR-ABL mutated clones prior to hematologic or cytogenetic resistance to imatinib. Haematologica 2008; 93: 186–192.
Tefferi A, Barosi G, Mesa RA, Cervantes F, Deeg HJ, Reilly JT et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood 2006; 108: 1497–1503.
Kroger N, Thiele J, Zander A, Schwerdtfeger R, Kobbe G, Bornhauser M et al. Rapid regression of bone marrow fibrosis after dose-reduced allogeneic stem cell transplantation in patients with primary myelofibrosis. Exp Hematol 2007; 35: 1719–1722.
Steensma DP . JAK2 V617F in myeloid disorders: molecular diagnostic techniques and their clinical utility: a paper from the 2005 William Beaumont Hospital Symposium on Molecular Pathology. J Mol Diagn 2006; 8: 397–411.
Fiorini A, Reddiconto G, Farina G, Marietti S, Palladino M, Chiusolo P et al. Eradication of JAK2 V617F mutation after allogeneic transplantation in a patient with myelofibrosis with myeloid metaplasia. Leukemia 2006; 20: 2198–2199.
Ruiz-Arguelles GJ, Garces-Eisele J, Reyes-Nunez V, Ruiz-Delgado GJ, Rosillo C, Camoriano JK . Clearance of the Janus kinase 2 (JAK2) V617F mutation after allogeneic stem cell transplantation in a patient with myelofibrosis with myeloid metaplasia. Am J Hematol 2007; 82: 400–402.
Koren-Michowitz M, Shimoni A, Vivante A, Trakhtenbrot L, Rechavi G, Amariglio N et al. A new MALDI-TOF-based assay for monitoring JAK2 V617F mutation level in patients undergoing allogeneic stem cell transplantation (allo SCT) for classic myeloproliferative disorders (MPD). Leuk Res 2008; 32: 421–427.
Steckel NK, Koldehoff M, Ditschkowski M, Beelen DW, Elmaagacli AH . Use of the activating gene mutation of the tyrosine kinase (Val617Phe) JAK2 as a minimal residual disease marker in patients with myelofibrosis and myeloid metaplasia after allogeneic stem cell transplantation. Transplantation 2007; 83: 1518–1520.
Levine RL, Wernig G . Role of JAK-STAT Signaling in the pathogenesis of myeloproliferative disorders. Hematology Am Soc Hematol Educ Program 2006, 233–239.
Pardanani AD, Levine RL, Lasho T, Pikman Y, Mesa RA, Wadleigh M et al. MPL515 mutations in myeloproliferative and other myeloid disorders: a study of 1182 patients. Blood 2006; 108: 3472–3476.
Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270.
Lo CF, Diverio D, Falini B, Biondi A, Nervi C, Pelicci PG . Genetic diagnosis and molecular monitoring in the management of acute promyelocytic leukemia. Blood 1999; 94: 12–22.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bacher, U., Zander, A., Haferlach, T. et al. Minimal residual disease diagnostics in myeloid malignancies in the post transplant period. Bone Marrow Transplant 42, 145–157 (2008). https://doi.org/10.1038/bmt.2008.185
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.185
Keywords
This article is cited by
-
Relapse assessment following allogeneic SCT in patients with MDS and AML
Annals of Hematology (2014)
-
CD34+ lineage specific donor cell chimerism for the diagnosis and treatment of impending relapse of AML or myelodysplastic syndrome after allo-SCT
Bone Marrow Transplantation (2013)
-
Prognostic and therapeutic implications of minimal residual disease at the time of transplantation in acute leukemia
Bone Marrow Transplantation (2013)
-
Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: results of the RELAZA trial
Leukemia (2012)
-
Allogeneic Hematopoietic Cell Transplantation for Myelodysplastic Syndrome: Current Status
Archivum Immunologiae et Therapiae Experimentalis (2012)