Abstract
Background:
In human papillomavirus (HPV)-based screening, a repeat HPV test is often recommended for HPV-positive women with normal cytology (HPV-pos/cyt-neg), but its absolute risk of cervical precancer (CIN3+) over two screening rounds needs to be assessed.
Methods:
We compared the 5-year risk of HPV infection and CIN3+ in HPV-pos/cyt-neg women with a negative repeat HPV test to the risk in HPV-negative women with normal cytology (double negatives) in the POBASCAM cohort. We obtained histology data from the Dutch pathology registry (PALGA).
Results:
Human papillomavirus infection risk was 20.4% (19 of 93) in HPV-pos/cyt-neg, repeat HPV-negative women and 3.2% (294 of 9186; P<0.001) in double negatives. Corresponding CIN3+ risks were 2.0% (4 of 199) and 0.2% (41 of 18 562; P<0.001). Infection risks were also increased in type-specific analyses of HPV16, 31, 33, 39, 52, 56 and 58.
Conclusions:
HPV-pos/cyt-neg women continue to have an increased CIN3+ risk, also when the repeat HPV test is negative. Therefore, intervals in primary HPV screening should be determined separately for HPV-positive and -negative women.
Similar content being viewed by others
Main
Human papillomavirus (HPV) testing provides better protection against cervical cancer and high-grade cervical intraepithelial neoplasia (CIN) than cytology (Arbyn et al, 2012; Ronco et al, 2014). Consequently, in several countries cytology is replaced by HPV (DNA) as primary screening test. Only a small proportion of HPV-positive women have cervical disease. To reduce the number of referrals, adjunct testing is required to detect the subset of HPV-positive women with CIN grade 3 or worse (CIN3+). However, there is still no general consensus about the most suitable triage strategy.
Options for stratification of HPV-positive women include reflex cytology, HPV16/18-genotyping and repeat HPV testing (Wright et al, 2011; Rijkaart et al, 2012a; Dijkstra et al, 2014). Post hoc evaluations of data collected within one screening round indicate that repeat HPV testing is associated with a high number of colposcopy referrals (Naucler et al, 2009; Rijkaart et al, 2012a; Dijkstra et al, 2014). The absolute CIN3+ risk after a negative repeat HPV test is assumed to be low, but can only be assessed with data from two screening rounds as many women with a negative repeat HPV test are referred to routine screening.
In this study, we compare the 5-year risk of HPV infection and CIN3+ in HPV-positive women with normal cytology (HPV-pos/cyt-neg) and a negative repeat HPV test to the risk in HPV-negative women with normal cytology (double negatives). We used data from the intervention group of the POBASCAM (Population Based Screening Study Amsterdam) cohort (Bulkmans et al, 2007; Rijkaart et al, 2012b) in which women were screened with both HPV and cytology in two rounds 5 years apart.
Materials and methods
Study population and procedures
The POBASCAM trial (Trial registration ID: NTR218) was designed to assess whether HPV testing in the first screening round decreases detection of CIN3 and cervical cancer in the second screening round (Bulkmans et al, 2007; Rijkaart et al, 2012b). Women aged 29–61 years old were randomised (1 : 1) to cytology and HPV co-testing (intervention group) or cytology only (control group). The intervention group of the POBASCAM trial consists of 19 999 eligible women (⩽56 years of age, no hysterectomy, no abnormal cytology in the preceding two years, valid HPV test). Thirty-three women without valid cytology and 680 women with abnormal cytology were excluded, leaving 19 286 women with normal cytology. In the second round after five years, women were managed according to the intervention group protocol. A detailed description of the management is available (Bulkmans et al, 2007; Rijkaart et al, 2012b). The POBASCAM trial was approved by the Medical Ethics Committee of the VU University Medical Centre (Amsterdam, the Netherlands; no 96/103) and the Ministry of Public Health (The Hague, the Netherlands; VWS no 328650). All participants provided written informed consent.
The HPV test (GP5+/6+-PCR EIA) detects 14 HPV types (16/18/31/33/35/39/45/51/52/56/58/59/66/68) and was done blinded to cytology (Jacobs et al, 1997; Bulkmans et al, 2004). Human papillomavirus -positive samples were typed by a reverse line blot assay (Van den Brule et al, 2002).
At colposcopy visit, biopsies were taken from suspected areas (Hopman et al, 1995, 2000). Histological examination was done locally and samples were classified as CIN0, CIN1, CIN2, CIN3 or invasive cancer (Anderson, 1995). Adenocarcinoma in situ was added to CIN3. Cytology and histology were identified through the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA) (Casparie et al, 2007). Follow-up results were encrypted and linkage was conducted based on last name, year of birth, enrolment cytology registry number, date of sample collection and laboratory.
Statistical analysis
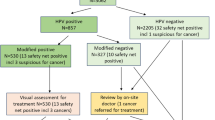
HPV-pos/cyt-neg women with a negative repeat HPV test were compared with double (HPV and cytology)-negative women (Figure 1). A negative repeat HPV result was defined as an HPV-negative test result at first repeat test scheduled at 6 months. In additional analyses, the subgroup of HPV-pos/cyt-neg women with an HPV-negative test result was extended with women with a positive repeat HPV test at 6 months followed by a negative repeat HPV test at 18 months. Five-year risk of HPV infection and CIN3+/2+ were compared using Fisher’s exact and the Mantel–Haenszel test, the latter one adjusting for age differences. Risk ratios (RR) were calculated. Analyses were performed with SPSS version 22.
Flowchart of women in the POBASCAM intervention group with normal cytology, including information on HPV repeat testing, HPV result at second screen and histology. Aden. Ca.=adenocarcinoma; CIN(2+)=cervical intraepithelial neoplasia (grade 2 or worse); HPV=human papillomavirus; SCC=squamous cell carcinoma.
Five-year risk of HPV infection was based on HPV results at the second screening round. A screening test was assigned to the second screen when taken between 4 and 9 years after enrolment. Screening results and histology occurring more than 9 years after enrolment were excluded. For the HPV infection risk analysis, women with CIN2+ or uterus extirpation before 4 years were excluded. HPV infection risk was calculated separately for all 14 HPV types.
Results
Study cohort characteristics
Seven hundred twenty-four out of 19 286 (3.8%) women with normal cytology had a positive HPV result at the baseline screen, 199 of whom had a negative HPV result at the first repeat test (Figure 1). Mean age was 37.9 (range 29–55). Mean time to first repeat HPV test was 9.8 months (range 3.0–29.7). Fifty-seven HPV-pos/cyt-neg women with an HPV-positive result at the first repeat test had an HPV-negative result at the second repeat test (time from baseline to second repeat test: 19.9 months, range 12.0–27.6). Mean age of 18 562 women with a negative HPV result at baseline screen was 41.4 (range 29–56).
HPV infection risk
Women without an HPV result at the second screening round (n=9473) and women with a CIN2+ or hysterectomy at baseline (n=9) were excluded, leaving 93 HPV-pos/cyt-neg women with a negative first repeat HPV result and 9186 HPV-neg/cyt-neg women (Figure 1). HPV-neg/cyt-neg women without an HPV result at the second screening round were slightly older than those with an HPV result (mean age: 41.8 vs 40.9; P<0.01). Among HPV-pos/cyt-neg, repeat HPV-negative women, age did not differ between those with and without an HPV result at the second screening round. Mean time from baseline to second screen was 59.9 months (range 48.1–81.6) in HPV-pos/cyt-neg women with an HPV-negative repeat test, and 60.8 months (range 48.0–102.8) in double negatives. HPV-pos/cyt-neg women who tested HPV-negative at the first repeat test had a 20.4% (19 of 93) HPV infection risk at the second screen 5 years later (Table 1, left columns). In comparison, HPV infection risk in double-negative women was 3.2% (294 of 9186) and significantly lower (RR 6.4; P<0.001). After correction for age, the relative risk remained statistically significant (RR 10.8; P<0.001). In 10 of 16 women with valid HPV genotypes in the baseline and second screening round, the HPV types detected in the second round were also found at baseline. In HPV-pos/cyt-neg women who tested HPV-negative at the first or second repeat test, similar relative risks were obtained (Table 1, right columns).
HPV16-pos/cyt-neg women with an HPV16-negative repeat test had a 5-year HPV16 infection risk of 17.6%, compared with 0.8% in HPV16-neg/cyt-neg women (RR 22.1; P<0.001). Type-specific infection risks were also increased for HPV type 31, 33, 39, 52, 56 and 58 (Table 1, left columns).
CIN3+ and CIN2+ risk
Thirty-two (16.1%) of 199 HPV-pos/cyt-neg women with a negative repeat HPV result and 875 out of 18 562 (4.7%) double negatives had a histology diagnosis within 9 years (Figure 1). Mean time from baseline to histology diagnosis was 52.4 months (range 0.03–107.4). Among 57 HPV-pos/cyt-neg women with an HPV-negative result at second repeat test, 11 (19.3%) had a histology diagnosis within 9 years (mean time from baseline to histology diagnosis was 51.7 months, range 5.6–86.2). Cervical intraepithelial neoplasia grade 3+ and CIN2+ risks are shown in Table 2. Cumulative CIN3+ risk in HPV-pos/cyt-neg women with a negative first repeat HPV result was 2.0%, and significantly higher than the 0.2% risk in double-negative women (RR 9.1, P<0.001). For CIN2+, risks were 5.5% and 0.5% (RR 11.7, P<0.001), respectively.
In six HPV-pos/cyt-neg women with a negative repeat HPV result and CIN2+, HPV typing results were available in the sample prior to CIN2+ (16, 31, 33, 58, 59, and multiple infection 18 of 52). The same HPV types were found in the corresponding baseline sample.
Discussion
The results show that HPV-pos/cyt-neg, repeat HPV-negative women have a significantly higher risk of overall and type-specific HPV infection and CIN3+ compared with double negatives. There are at least two possible explanations for the alternating HPV-positive, -negative, -positive pattern. When the new infection is of a different type than found in the baseline round, the pattern reflects viral clearance followed by a newly acquired infection. In our study, this only holds for a minority of the infections and only those without CIN2+. When the same HPV type is observed in the baseline and second round, the pattern may still reflect reinfection as natural immunity after clearance offers only limited protection (Trottier et al, 2010), but it may also be caused by a temporary decrease in viral load below the detection threshold (Woodman et al, 2001; Insinga et al, 2010; Maglennon et al, 2011; Rositch et al, 2012). The latter explanation is important for defining screening algorithms. It suggests that once infected, women remain at increased HPV and CIN3+ risk, also after a negative repeat HPV test, and that screening intervals in primary HPV screening programmes should be determined separately for HPV-positive and -negative women.
A limitation of the POBASCAM study is that histology diagnosis was done by local pathologists. However, interobserver reliability of CIN3+ was very high (Bulkmans et al, 2004). Another limitation is that follow-up screening was incomplete, mainly because only a cytological assessment was requested by the general practitioners in accordance with the standard screening guidelines in the Netherlands at that time. Attendance in the second round was 88% in HPV-pos/cyt-neg, repeat HPV test negative women and 85% in double negatives. Second round HPV was missing in 53.3% and 50.4% in the two strata, respectively. Together, this means that the incomplete follow-up may negatively bias CIN risk estimates, but similarly in the two study strata.
The results of this study show that HPV-pos/cyt-neg women continue to have an increased CIN3+ risk, also when the repeat HPV test is negative. Therefore, intervals in primary HPV screening should be determined separately for HPV-positive and -negative women, and a triage testing algorithm should optimally weigh CIN3+ risk and colposcopy referral rate. In our viewpoint, a triage strategy that uses repeat cytology seems appropriate as the colposcopy referral rate will be approximately 40% lower in comparison with repeat HPV testing (Rijkaart et al, 2012a; Dijkstra et al, 2014).
Change history
07 November 2017
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Anderson B (1995) Premalignant and malignant squamous lesions of the cervix. In:Fox H (ed). Haines and Taylor Obstetrical and Gynaecological Pathology. Churchill Livingstone: Edinburgh, Scotland, pp 273–322.
Arbyn M, Ronco G, Anttila A, Meijer CJ, Poljak M, Ogilvie G, Koliopoulos G, Naucler P, Sankaranarayanan R, Peto J (2012) Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 30: F88–F99.
Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ (2007) Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 370: 1764–1772.
Bulkmans NW, Rozendaal L, Snijders PJ, Voorhorst FJ, Boeke AJ, Zandwijken GR, van Kemenade FJ, Verheijen RH, v Groningen K, Boon ME, Keuning HJ, van Ballegooijen M, van den Brule AJ, Meijer CJ (2004) POBASCAM, a population-based randomized controlled trial for implementation of high-risk HPV testing in cervical screening: design, methods and baseline data of 44,102 women. Int J Cancer 110: 94–101.
Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA (2007) Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29: 19–24.
Dijkstra MG, van Niekerk D, Rijkaart DC, van Kemenade FJ, Heideman DA, Snijders PJ, Meijer CJ, Berkhof J (2014) Primary hrHPV DNA testing in cervical cancer screening: how to manage screen-positive women? A POBASCAM trial substudy. Cancer Epidemiol Biomarkers Prev 23: 55–63.
Hopman EH, Rozendaal L, Voorhorst FJ, Walboomers JM, Kenemans P, Helmerhorst TJ (2000) High risk human papillomavirus in women with normal cervical cytology prior to the development of abnormal cytology and colposcopy. BJOG 107: 600–604.
Hopman EH, Voorhost FJ, Kenemans P, Meyer CJ, Helmerhorst TJ (1995) Observer agreement on interpreting colposcopic images of CIN. Gynecol Oncol 58: 206–209.
Insinga RP, Perez G, Wheeler CM, Koutsky LA, Garland SM, Leodolter S, Joura EA, Ferris DG, Steben M, Brown DR, Elbasha EH, Paavonen J, Haupt RM FUTURE I Investigators (2010) Incidence, duration, and reappearance of type-specific cervical human papillomavirus infections in young women. Cancer Epidemiol Biomarkers Prev 19: 1585–1594.
Jacobs MV, Snijders PJ, van den Brule AJ, Helmerhorst TJ, Meijer CJ, Walboomers JM (1997) A general primer GP5+/6(+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol 35: 791–795.
Maglennon GA, McIntosh P, Doorbar J (2011) Persistence of viral DNA in the epithelial basal layer suggests a model for papillomavirus latency following immune regression. Virology 414: 153–163.
Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, Rådberg T, Strander B, Forslund O, Hansson BG, Hagmar B, Johansson B, Rylander E, Dillner J (2009) Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 101: 88–99.
Rijkaart DC, Berkhof J, Rozendaal L, van Kemenade FJ, Bulkmans NW, Heideman DA, Kenter GG, Cuzick J, Snijders PJ, Meijer CJ (2012b) Human papillomavirus testing for the detection of high-grade cervical intraepithelial neoplasia and cancer: final results of the POBASCAM randomised controlled trial. Lancet Oncol 13: 78–88.
Rijkaart DC, Berkhof J, van Kemenade FJ, Coupe VM, Hesselink AT, Rozendaal L, Heideman DA, Verheijen RH, Bulk S, Verweij WM, Snijders PJ, Meijer CJ (2012a) Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer 130: 602–610.
Ronco G, Dillner J, Elfström KM, Tunesi S, Snijders PJ, Arbyn M, Kitchener H, Segnan N, Gilham C, Giorgi-Rossi P, Berkhof J, Peto J, Meijer CJ International HPV screening working group (2014) Efficacy of HPV-based screening for prevention of invasive cervical cancer: follow-up of four European randomised controlled trials. Lancet 383: 524–532.
Rositch AF, Burke AE, Viscidi RP, Silver MI, Chang K, Gravitt PE (2012) Contributors of recent and past sexual partnerships on incident human papillomavirus detection: acquisition and reactivation in older women. Cancer Res 72: 6183–6190.
Trottier H, Ferreira S, Thomann P, Costa MC, Sobrinho JS, Prado JC, Rohan TE, Villa LL, Franco EL (2010) Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer Res 70: 8569–8577.
Van den Brule AJ, Pol R, Fransen-Daalmeijer N, Schouls LM, Meijer CJ, Snijders PJ (2002) GP5+/6+ PCR followed by reverse line blot analysis enables rapid and high-throughput identification of human papillomavirus genotypes. J Clin Microbiol 40: 779–787.
Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, Yates M, Rollason TP, Young LS (2001) Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 357: 1831–1836.
Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group (2011) Evaluation of HPV-16 and HPV-18 genotyping for triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 136: 578–586.
Acknowledgements
We thank the PALGA foundation for their help with the PALGA search strategy and PALGA data collection. The original POBASCAM study was supported by the Netherlands Organisation for Health Research and Development (Zorg Onderzoek Nederland; grant no 30-05220). This work was supported by EC FP7 Health 2013 Innovation 1 CoheaHr (grant no 603019) and ZonMW (grant no 531002007).
Disclaimer
The funders had no role in the study design, data collection, data analysis, data interpretation or writing of the report.
Ethics
The POBASCAM trial was approved by the medical ethics committees of the VU University Medical Centre (no 96/103) and the Ministry of Public Health (VWS no 328 650). All women have provided written informed consent before they were included in the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
NJV has received travel support from DDL Diagnostic Laboratory. DAMH serves occasionally on the scientific advisory boards of AMGEN and Pfizer. PJFS has been on the speakers bureau of Roche diagnostics, Gen-Probe, Abbott, Qiagen and Seegene and has been a consultant for Crucell B.V. CJLMM has received speakers fee from GSK, Qiagen, SPMSD/Merck, Roche diagnostics, Menarini and Seegene, served occasionally on the scientific advisory board (expert meeting) of GSK, Qiagen, SPMSD/Merck, Roche and Genticel and has been by occasion consultant for Qiagen and Genticel. CJLMM was minority shareholder of Diassay B.V. until April 2016, until 2014 he held a small number of certificates of shares in Delphi Biosciences, which went into receivership in 2014. DAMH, PJFS and CJLMM are minority shareholders of Self-screen B.V., a spin-off company of VUmc. Self-screen B.V. holds patents related to the work. JB has received consultancy fees from Roche, GlaxoSmithKline, and Merck/SPMSD and received travel support from DDL. All fees were collected by his employer. The remaining author declare no conflict of interest.
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/4.0/
About this article
Cite this article
Polman, N., Veldhuijzen, N., Heideman, D. et al. HPV-positive women with normal cytology remain at increased risk of CIN3 after a negative repeat HPV test. Br J Cancer 117, 1557–1561 (2017). https://doi.org/10.1038/bjc.2017.309
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2017.309
Keywords
This article is cited by
-
Validation of an on-chip p16ink4a/Ki-67 dual immunostaining cervical cytology system using microfluidic device technology
Scientific Reports (2023)
-
Factors associated with low adherence to cervical cancer follow-up retest among HPV+/ cytology negative women: a study in programmatic context in a low-income population in Argentina
BMC Cancer (2019)
-
Burden and genotype distribution of high-risk Human Papillomavirus infection and cervical cytology abnormalities at selected obstetrics and gynecology clinics of Addis Ababa, Ethiopia
BMC Cancer (2019)