Abstract
Background:
Although most epidemiological studies suggest that non-steroidal anti-inflammatory drug use is inversely associated with prostate cancer risk, the magnitude and specificity of this association remain unclear.
Methods:
We examined self-reported aspirin and ibuprofen use in relation to prostate cancer risk among 29 450 men ages 55–74 who were initially screened for prostate cancer from 1993 to 2001 in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Men were followed from their first screening exam until 31 December 2009, during which 3575 cases of prostate cancer were identified.
Results:
After adjusting for potential confounders, the hazard ratios (HRs) of prostate cancer associated with <1 and ⩾1 pill of aspirin daily were 0.98 (95% confidence interval (CI), 0.90–1.07) and 0.92 (95% CI: 0.85–0.99), respectively, compared with never use (P for trend 0.04). The effect of taking at least one aspirin daily was more pronounced when restricting the analyses to men older than age 65 or men who had a history of cardiovascular-related diseases or arthritis (HR (95% CI); 0.87 (0.78–0.97), 0.89 (0.80–0.99), and 0.88 (0.78–1.00), respectively). The data did not support an association between ibuprofen use and prostate cancer risk.
Conclusion:
Daily aspirin use, but not ibuprofen use, was associated with lower risk of prostate cancer risk.
Similar content being viewed by others
Main
Prostate cancer is the most common non-skin malignancy, accounting for an estimated 29% of all newly diagnosed cancers in 2012 among US men (Siegel et al, 2012). Despite the large morbidity, the aetiology of prostate cancer remains unclear, with only older age, African ancestry, family history of the disease, and several loci in the 8q24 region as established risk factors (Hsing and Chokkalingam, 2006; Witte, 2007; Cheng et al, 2008; Chu et al, 2008). Several lines of evidence also point to chronic inflammation of the prostate as a potential predisposing factor, including data suggesting that non-steroidal anti-inflammatory drug (NSAID) use can inhibit prostate carcinogenesis (Stock et al, 2008; De Nunzio et al, 2011). Non-steroidal anti-inflammatory drugs block the conversion of arachidonic acid to prostaglandins, which are key mediators of the inflammatory response, by inhibiting the enzyme cyclooxygenase (COX, also called prostaglandin synthase; Smith et al, 2000). Both COX-1 and COX-2 isoforms are expressed in the human prostate (O’Neill and Ford-Hutchinson, 1993), with multiple reports of elevated COX-2 expression in prostate adenocarcinoma relative to benign hyperplasia or normal tissue (Gupta et al, 2000; Hsu et al, 2000; Madaan et al, 2000; Lee et al, 2001; Uotila et al, 2001). Some studies have further correlated COX-2 expression with severe tumour grade (Madaan et al, 2000; Lee et al, 2001), whereas others have found COX-2 overexpression restricted to regions of prostatic proliferative inflammatory atrophy (Zha et al, 2001). In addition, treatment of prostate cancer cell lines with selective COX-2 inhibitors has been shown to induce apoptosis (Liu et al, 1998, 2000; Hsu et al, 2000; Kamijo et al, 2001), reduce angiogenesis (Liu et al, 2000), and inhibit cellular invasion (Attiga et al, 2000).
Although some epidemiological studies have shown inverse relationships between NSAID use and prostate cancer risk (Friis et al, 2003; Sorensen et al, 2003; Garcia Rodriguez and Gonzalez-Perez, 2004; Mahmud et al, 2004, 2006, 2011; Jacobs et al, 2005, 2007, 2011; Bosetti et al, 2006; Dasgupta et al, 2006; Liu et al, 2006; Cheng et al, 2007; Salinas et al, 2010; Dhillon et al, 2011), the risk reductions have been modest and only some, not all, have yielded statistically significant results (Nelson and Harris, 2000; Habel et al, 2002; Roberts et al, 2002; Perron et al, 2003; Garcia Rodriguez and Gonzalez-Perez, 2004; Jacobs et al, 2005, 2007, 2011; Dasgupta et al, 2006; Liu et al, 2006; Mahmud et al, 2006, 2011; Cheng et al, 2007; Dhillon et al, 2011). In addition, one study reported a significant elevated risk of prostate cancer in association with NSAID non-aspirin use (Murad et al, 2011). However, in a recent meta-analysis (Mahmud et al, 2010), the summary odds ratios for prostate cancer associated with aspirin use were 0.83 (95% confidence interval (CI): 0.77–0.89) for all studies and 0.81 (95% CI: 0.72–0.92) for advanced prostate cancer. The ORs associated with non-aspirin NSAID for all studies or advanced prostate cancer were 0.89 (95% CI: 0.78–1.08) and 0.98 (95% CI: 0.59–1.65), respectively. The lack of clear consistency across studies of NSAID use and prostate cancer risk may be explained by factors related to study design, including choice of study population, exposure definition and assessment, length of follow-up, prostate cancer detection, and confounding. The observed differences in cancer risk associated with aspirin relative to other NSAIDs may also be attributed in part to pharmacological differences between individual NSAIDs.
Therefore, to overcome the shortcoming of previous studies, we examined the relationship of aspirin and ibuprofen use with subsequent risk of prostate cancer in a cohort of 29 450 men participating in the screening arm of Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. The use of this cohort provided us with prospective data on a wide range of topics, including medical history, allowing for adjustment for several putative confounders. In addition, men in the intervention arm of the trial were uniformly screened for prostate cancer, therefore minimising misclassification of the outcome. Previous studies may have included occult cancers, which could cause participants to increase their NSAID use, thereby masking the protective effect of NSAID use due to reverse causality. The PLCO Cancer Screening Trial, on the other hand, included few prevalent cancers owing to its nature as a screening trial. Also, given the large number and diversity of participants from 10 screening centres across the United States, the PLCO Trial presented an opportunity to further assess the relationship between NSAID use and prostate cancer risk within a more broadly representative sample of the US population than most previous studies. In addition, PLCO’s use of active and passive surveillance and cause of death review process allowed the most comprehensive ascertainment of study endpoints including prostate cancer and death (Prorok et al, 2000).
Methods
The PLCO Cohort detailed information on the PLCO Trial has been published elsewhere (Gohagan et al, 2000; Prorok et al, 2000). Approval of the trial protocol and procedures was granted by the Institutional Review Boards of the National Cancer Institute and the 10 screening centres (Birmingham, AL, USA; Denver, CO, USA; Detroit, MI, USA; Honolulu, HI, USA; Marshfield, WI, USA; Minneapolis, MN, USA; Pittsburgh, PA, USA; Salt Lake City, UT, USA; St Louis, MO, USA; and Washington, DC, USA). All participants provided written informed consent. Study eligibility for men was restricted to those ages 55–74 who were not under current treatment for any cancer (except basal and squamous cell skin cancer); had no prior history of prostate, lung, or colorectal cancer; had not undergone surgical removal of a lung or the prostate or colon; had not taken Proscar (Finasteride) in the past 6 months; and were not already enrolled in another cancer screening or prevention trial. This study was further limited to men randomised to the screening arm.
At study entry, men were screened for prostate cancer by digital rectal examination (DRE) and prostate-specific antigen (PSA) testing. They also completed a brief, structured questionnaire on risk factors for cancer and a 137-item food frequency questionnaire on diet and nutrient supplement use in the last 12 months. Screening for prostate cancer occurred annually for 5 years after the initial visit. Digital rectal examination was performed in the first 3 years, while serum PSA levels were tested for 5 consecutive years. Men with suspicious findings for prostate cancer (i.e., PSA >4 ng ml−1 or DRE with nodularity, indurations, or asymmetry of the prostate gland) were referred for further diagnostic evaluation. Medical record review was conducted by trained abstractors to capture data on relevant diagnostic and therapeutic procedures that occurred up to 1 year after cancer detection. Also as part of the follow-up process, participants were mailed annual surveys that were used to ascertain cancer incidence and death, and loss to follow-up was established based upon failure to receive these completed surveys. For completeness of follow-up, where available, prostate cancer data were retrieved from the population-based cancer registries serving the relevant study centre. Additional ascertainment of vital status was conducted using the National Death Index. Underlying cause of death was determined for all participants by unbiased death review panels that were blinded to the study arm and not affiliated with the study centre. The death review process entails reviewing the death certificates, medical reports, and autopsy reports. Pathological grade was assessed using the biopsy/resection Gleason score (range 2–10). Clinical staging of patients were determined using the TNM staging system (Fleming et al, 1997). An aggressive cancer was defined as a Gleason score of 7 or higher, or a stage III or IV case.
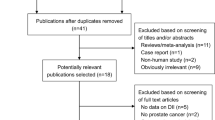
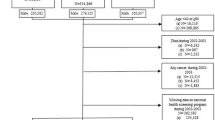
Of the 38 340 men randomised to the screening arm, we focused our study on the 37 448 who had NSAID use data and who were first screened for prostate cancer between November 1993 and July 2001. Men were excluded from the analysis if they (1) reported a prior history of cancer at baseline, except non-melanoma skin cancer (n=1726); (2) failed to complete the baseline risk factor (n=892) and dietary (n=6586) questionnaires; (3) had no further contact (i.e., no follow-up) after the baseline screening exam (n=4107); and (4) had an insufficient dietary assessment (7452) or 5) did not have adequate baseline PSA (n=4115). Men who refrained from answering any question pertaining to NSAID use (n=913) were additionally excluded (counts are not mutually inclusive), resulting in a final study cohort of 29 450 men, with 3575 cases of prostate cancer identified in subsequent follow-up. There were 546 cancers diagnosed within the first year of follow-up and 3029 cancers diagnosed after the first year of follow-up.
Assessment of NSAID use
The baseline questionnaire included four questions that pertained to aspirin and ibuprofen use: (1) During the last 12 months, have you regularly used aspirin or aspirin-containing products, such as Bayer, Bufferin, or Anacin? (Specific instruction was provided to not count aspirin-free products, such as Tylenol or Panadol). (2) During the last 12 months, how many pills of aspirin or aspirin-containing products did you usually take per day, per week, or per month? (3) During the last 12 months, have you regularly used ibuprofen-containing products, such as Advil, Nuprin, or Motrin? and (4) During the last 12 months, how many pills of ibuprofen-containing products did you usually take per day, per week, or per month? Possible responses for frequency of use of aspirin or ibuprofen included none, <2 per month, 2–3 per month, 1 per week, 2 per week, 3–4 per week, 1 per day, and 2 or more per day.
Statistical analysis
In our analysis, men were categorised separately for each NSAID according to their reported frequency of use at baseline: never use, 1–29 pills per month (<1 pill per day), 1 pill per day, and ⩾2 pills per day. We also considered whether aspirin and ibuprofen were used jointly or separately by creating another variable with categories of neither, only regular aspirin use, only regular ibuprofen use, and regular use of both NSAIDs.
Hazard ratios (HRs) and 95% CIs for prostate cancer related to the frequency of aspirin and ibuprofen use were estimated by Cox regression with the HR modelled as a function of age (Korn et al, 1997). The follow-up period for each individual began from the individual age at the baseline screening until the individual age at the prostate cancer diagnosis, death, loss to follow-up, or the administrative censor date (31 December 2009), whichever occurred first. To examine dose response effects associated with increasing frequency of use, trend tests were performed on a continuous scale, where each category was assigned the value of 0, 1, …, and so on, and included in the model as a continuous variable. Multivariate analyses were conducted to evaluate potential confounding by age (modelled as the underlying metric/time, that is, not as a covariate in the model); race (White, Black, Asian/Pacific Islander, other); study centre; family history of prostate cancer (yes, no); the number of screening exams in the follow-up period; education (less than high school, high school graduate, some college, college graduate, or higher); smoking status (never, former cigarette use, current cigarette use, pipe, or cigar use); baseline body mass index (BMI, <25.0, 25.0–29.9, ⩾30 kg m−2); physical activity (none, <1, 1, 2+ hours per week); total energy (kcal per day, in quintiles); various dietary factors (grams per day, in quintiles, adjusted for energy using the residual method (Willett and Stampfer, 1986)), including total β-carotene, vitamin C, vitamin A, vitamin D, vitamin E, calcium, fat, red meat, and lycopene; and self-reported history of various medical conditions (yes or no), including hypertension, heart attack, stroke, diabetes, arthritis, and colon polyps. Final multivariate models were adjusted for age, race, study centre, family history of prostate cancer, the number of screening exams, aspirin use (for ibuprofen), and ibuprofen use (for aspirin). A variable remained in the final model if it resulted in >10% change in the HR estimate of NSAID. Calculated P-values were two-sided, with P<0.05 considered statistically significant.
To address the probability of reverse causality (i.e., individuals with occult cancer might experience pain that lead to the use of NSAID), which may lead to variation in the risk associated with NSAID use in cancers diagnosed within the first year of follow-up and cancers diagnosed after the first year of follow-up, analyses were performed to estimate the risk of cancers diagnosed within the first year of follow-up and cancers diagnosed after the first year of follow-up in association with aspirin and ibuprofen use. The significance of the difference in risk by time of cancer diagnosis was tested by including an interaction term as cross product between aspirin/ibuprofen and time of cancer diagnosis (as a dummy variable of first year vs later years). Hazard ratios for non-aggressive and aggressive prostate cancers were also estimated, such that for aggressive cancer, analysis was restricted to cases with aggressive cancer compared with the controls. Similarly for non-aggressive cancers analysis was restricted to cases with non-aggressive cancers and the controls. Prostatic tumours classified as stage III or IV or designated Gleason scores of 7 or higher were defined as aggressive (n=1560), whereas all other tumours were defined as non-aggressive (n=2015). To address the possibility that death may act as a competing risk, we conducted sensitivity analysis where death was considered as a competing risk instead of being a censoring variable (Andersen, 1993). Stratified analyses were conducted to determine whether HRs associated with NSAID use varied by age (<65, ⩾65 years) and by history of selected medical conditions (yes and no). Risk differences by race could not be examined as 92.4% of the study population was White. Individuals with missing data on any variable were excluded in the analysis.
Results
In this cohort, the mean age at entry was 62.8 (s.d.=5.3) years, with a median (interquartile range) follow-up time of 11.7 (9.5,12.9) years. Most men (>90%) were Caucasian and college educated. The cohort included 25 875 controls and 3575 prostate cancer cases. During the study follow-up, 105 prostate cancer-related deaths and 4708 other causes deaths were observed, with a median (interquartile range) follow-up time of 5.0 (2.0–8.5) in cases and 12.2 (10.3, 12.9) in controls. The age and race of men who did not respond to the NSAID questions were not significantly different from responders. In the following analyses, the use of aspirin or ibuprofen was assessed at the study entry. For simplicity, in the remaining of the manuscript, we will refer to these exposures as aspirin and ibuprofen use. On a daily basis, aspirin was used more than ibuprofen, with only 1.2% of men taking both drugs. For aspirin, 46% reported never use and 30.7% reported use of ⩾1 pill per day, whereas for ibuprofen, 75.2% reported never use and 7.6% reported use of ⩾1 pill per day.
As shown in Table 1, daily use of aspirin was greater with age: 26.6% for <60 years and 36.3% for ⩾70 years. Although daily use of ibuprofen was fairly consistent across age groups, the oldest men used ibuprofen least often. Daily use of either aspirin or ibuprofen was much less common among Asian/Pacific Islander men than other racial groups. Frequency of NSAID use, however, did not differ substantially by education or family history of prostate cancer. There was also minimal variation in NSAID use by smoking status, although use was most prevalent among current cigarette smokers. Use of both aspirin and ibuprofen was greatest among men with a baseline BMI of ⩾30 kg m−2. The prevalence of daily aspirin use was slightly higher with greater physical activity, whereas the prevalence of daily ibuprofen use was lower. Daily aspirin use was greatest among men with a history of cardiovascular-related conditions, including heart attack (72.5%), stroke (60.2%), and hypertension (41.4%), and with a history of diabetes (43.4%), whereas daily ibuprofen use was greatest among men with a history of arthritis (14.9%).
The daily use of aspirin only was significantly associated with lower risk of cancer HR (95% CI) of 0.91 (0.84–0.99). Although the daily use of ibuprofen only showed similar trend, however, it was not statistically significant 0.88 (0.65–1.20). Interestingly, the daily use of aspirin plus ibuprofen was associated with excess risk of prostate cancer 1.55 (1.13–2.13).
In multivariable analysis, the risks for prostate cancer associated with taking
History of various medical conditions including; diabetes, hypertension, stroke, heart attack, arthritis, and colon polyp did not appreciably modify the association between the use of either NSAID and prostate cancer risk (data not in tables). However, in stratified analysis, the inverse associations appeared more pronounced among men taking at least one aspirin daily who were older than 65 years with a HR (95% CI) of 0.87 (0.78–0.97) and who reported having a history of cardiovascular-related diseases HR (95% CI)=0.89 (0.80–0.99) or arthritis HR (95% CI)=0.88 (0.78–1.00) despite the lack of significant interaction.
Discussion
Data from this prospective cohort study suggest that regular use of aspirin, but not ibuprofen, was associated with a reduced prostate cancer risk. Overall decreases in prostate cancer risk associated with regular aspirin use were modest, although there was some evidence of further risk reduction with more frequent use, particularly among men who were older than age 65 or who had a prior history of cardiovascular-related diseases or arthritis.
The lack of an association between ibuprofen use and prostate cancer risk in this analysis is likely attributed in part to the low prevalence of ibuprofen use in the PLCO cohort. Previous studies of ibuprofen have conflicting results. Although few studies reported reduced risk of prostate cancer in association with non-aspirin NSAID use (Dasgupta et al, 2006; Mahmud et al, 2011), some studies reported increased (Murad et al, 2011) or no risk (Platz et al, 2005; Brasky et al, 2010). Laboratory data indicate that both aspirin and ibuprofen can inhibit prostatic carcinogenesis (Attiga et al, 2000; Andrews et al, 2002; Lloyd et al, 2003). In a study that compared the effect of various non-prescription NSAIDs (including aspirin and ibuprofen) on prostate tumour cell survival, ibuprofen was the most effective in suppressing proliferation and inducing apoptosis, particularly at clinically prescribed doses (Andrews et al, 2002).
Owing to the potential adverse effects of aspirin, such as gastrointestinal tract and renal toxicity, it is useful to identify subgroups of men for whom use of aspirin is particularly beneficial. In analyses restricted to men with a history of cardiovascular-related diseases and men with a history of arthritis, we observed inverse associations between frequency of aspirin use and prostate cancer risk. Although the questionnaire lacked data on specific dose and duration of NSAID use, our results of lower risk among men older than 65 years and among those who have history of cardiovascular diseases and arthritis suggest that the reduction in prostate cancer risk is likely conferred by aspirin taken at generally low doses (such as for cardiovascular disease) for long duration (among older men). This notion is further supported by the current common medical practice, where for the purpose of coronary artery disease prevention doctors prescribe the lower dose aspirin (81 mg day−1) for the majority of patients (about 60%) and regular dose aspirin (325 mg day−1) for about 35% (Campbell et al, 2007)).
Several sources of bias, including confounding and detection bias, could have affected the observed associations of aspirin and ibuprofen use with prostate cancer risk. Men who take NSAIDs daily for preventive purposes may be more health conscious and more likely to engage in positive health-related behaviours, such as maintaining a healthy diet and exercising regularly, which could influence their risk for prostate cancer. However, controlling for physical activity, dietary fat consumption, and other dietary factors suspected to decrease prostate cancer risk, including lycopene and vitamin E intake, did not materially alter the risk estimates for either NSAID. Confounding may further exist if NSAID use is related to a physical condition that directly affects prostate cancer risk (Psaty et al, 1999). Although adjustment for several factors, including arthritis and hypertension, did not alter the results, residual confounding by unknown factors cannot be ruled out.
The intriguing association between aspirin use and prostate cancer risk among men with cardiovascular-related diseases merits further investigation. Reasons for the inverse relation between aspirin use and prostate cancer risk observed among men with cardiovascular-related diseases are unknown, but recent studies suggest that prostate cancer and cardiovascular disease share common risk factors, such as hyperlipidemia and chronic inflammation, and that regular statin use lowers the risk of prostate cancer (Platz et al, 2006; Flick et al, 2007; Jacobs et al, 2007; Taylor et al, 2008). It should be noted that the prevalence of daily aspirin use was greater among men who reported a history of cardiovascular-related diseases or diabetes than men without these conditions. In the PLCO cohort, diabetes, a major risk factor for cardiovascular disease, had divergent relations with prostate cancer by tumour aggressiveness. In these men, diabetes was associated with a reduced risk of total prostate cancer but an increased risk of aggressive prostate cancer among men who were lean or physically active (Leitzmann et al, 2008). Additional adjustment for history of diabetes, however, did not materially change the association between aspirin use and prostate cancer risk in our analysis.
Surveillance bias is possible but not likely to account for the suggestive association between aspirin use and prostate cancer risk. In theory, NSAID users, being either more health conscious or more burdened with other medical problems, may be under closer medical surveillance than non-users, thereby increasing their chances for early detection of prostate cancer. Such bias, if any, is likely minimal in our study, as men participating in the screening arm of the PLCO Trial had an equal opportunity for cancer detection – a unique difference from prior studies – with screening visits scheduled annually over the first 5 years of follow-up. To account for any residual difference in adherence to annual screening between NSAID users and non-users, the total number of screening visits across the follow-up period for each individual was treated as a confounding variable (Weiss, 2003), but it yielded no substantial change in the magnitude or direction of risk associated with NSAID use.
Although recall bias was minimised by the prospective study design, exposure misclassification could have occurred if there were any changes in NSAID use related to symptoms of undiagnosed prostatic disease within the last 12 months before enrolment. This would be most likely to affect those men diagnosed with prostate cancer earlier in the follow-up period. Although excluding the 15% of cases who were diagnosed within the first year of follow-up (i.e., probably prevalent cases) from the analysis did weaken the association between daily aspirin use and prostate cancer risk, there was no meaningful difference in the HRs for cancers diagnosed within the first year of follow-up and cancers diagnosed after the first year of follow-up. Another unresolved issue is whether NSAIDs are more effective at inhibiting tumour progression than initiation. Two studies have suggested that frequent aspirin use reduces risk for advanced or metastatic prostate cancer (Norrish et al, 1998; Leitzmann et al, 2002). In contrast, another cohort study showed about a 24% reduction in prostate cancer risk with daily aspirin use, the magnitude of which did not differ between local and regional/distant disease (Habel et al, 2002). Given that our male cohort originated from the screening arm of the PLCO Trial, participants were screened annually. Therefore, cancer cases were more likely to be caught early. Thus, few cases of metastatic cancer were diagnosed, with almost half of the metastatic cancer cases detected within 1 year of the initial screening visit. Under these circumstances, only regular aspirin use in relation to advanced prostate cancer could be examined. We found a slightly lower risk for aggressive compared with non-aggressive tumours, indicating that aspirin might be more influential in hindering the progression than development of prostatic tumours.
Even though the underlying mechanisms have yet to be precisely delineated, the potential benefit of aspirin does support the prevailing hypothesis that chronic inflammation contributes to prostate carcinogenesis. As a response in the repair of damaged or infected prostatic tissue, chronic inflammation may promote neoplastic development and growth by triggering specific cytokines and growth factors, activating COX-2 in macrophages and epithelial cells, and inducing oxidative stress (Lucia and Torkko, 2004). Accordingly, increasing attention has been devoted to the focal lesions of epithelial atrophy associated with chronic inflammation and a high proliferative index, collectively known as proliferative inflammatory atrophy. These lesions have been commonly observed in the peripheral zone of the prostate where most tumours originate, found in close proximity to both adenocarcinoma and high-grade prostatic intraepithelial neoplasia, and associated with COX-2 upregulation (Zha et al, 2001; Platz and De Marzo, 2004).
Despite numerous strengths, including low attrition, nearly complete histological confirmation of cancer cases, comprehensive baseline data on potential confounders, and equal access for prostate cancer screening among participants, this study had several limitations. Data on frequency of aspirin and ibuprofen use were collected at a single point in time (during the last 12 months), without record of dose (pill count), duration, or indication for use. Therefore, there might be some misclassification of NSAID use because the assessment relied only on baseline self-reports. However, although there might be an underestimation because people tend to increase the frequency or the dose of use of NSAID by age, this underestimation is expected to be non-differential because the reporting was before the development of cancer. In fact this underestimation, if it biased our results at all, would bias the results towards the null. Thus, our estimates are conservative. In addition, misclassification might have occurred because of the use of some prescription and/or over-the-counter NSAID that the respondent might overlooked. However, if there is misclassification, it would most likely be non-differential, therefore potentially attenuating our results.
The prevalence of regular aspirin use in this study (31%), nevertheless, was comparable to that noted in the other US-based cohort studies of prostate cancer, which ranged from 17% (for daily use) to 59% (for use in the past month; Paganini-Hill et al, 1989; Schreinemachers and Everson, 1994). In a US study on patterns of aspirin use among adults ages 45–64, the prevalence of aspirin use among white men was 31%, with increasing trends in prevalence noted both across the study period (1987 to 1989) and with older age. In addition, a recent report from the Household Component of the Medical Expenditure Panel Survey by the Agency for Health Care Research and Quality concluded that 19.3% of the adult US non-institutionalised individuals report aspirin use either daily or every other day. The use increases by age, and almost 50% of individuals older than 65 years old reported daily use (Soni, 2007). Exposure assessment was also limited by not taking into account the use of other specific NSAIDs and by not verifying self-reported data on NSAID use through medical record review. However, misclassification due to the use of COX-2 selective inhibitors, such as celecoxib and rofecoxib, was probably minimal, as these ‘new generation’ NSAIDs were first introduced in 1999, which was toward the end of the trial recruitment period. Although the extent and impact of exposure misclassification cannot be determined, extensive measurement error would most likely lead to risk attenuation.
In addition, as the majority of the participants are White, the results might not be generalisable to other races. Another limitation of this type of data is left truncation, such that participants who enter the cohort at a certain age have obviously not died or developed cancer before that age. Subjects who died or developed cancer before enrolment would clearly not be included in the cohort, thus resulting in left truncation. However, we used PROC PHREG in SAS/STAT software (Version 9.2, SAS Institute Inc., Cary, NC, USA), which allows late entry models. This analysis method handles the left truncated data therefore alleviates this limitation.
Another limitation that should be noted is the competing mortality due to aging or other medical conditions. However, in the competing risk sensitivity analysis, the inverse associations we observed between NSAID use and prostate cancer are unlikely to be due to an increased risk of death among men who take NSAIDs; since the results were essentially the same for the analysis with and without death included as a competing risk.
In summary, this prospective cohort study suggests that aspirin use was associated with a reduced prostate cancer risk, in particular, in certain subgroups of men. Additional studies with more detailed exposure measurement are warranted to evaluate the dose, duration, and timing of NSAID use in relation to prostate cancer risk. The association between non-selective and selective COX-2 inhibitors, as well as non-COX inhibitors, should also be investigated. Coupled with laboratory-based research, these efforts should further expand our knowledge of the mechanisms by which NSAIDs, particularly aspirin, may influence prostate carcinogenesis.
Change history
25 June 2012
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Andersen PK (1993) Statistical Models Based on Counting Processes. Springer-Verlag: New York
Andrews J, Djakiew D, Krygier S, Andrews P (2002) Superior effectiveness of ibuprofen compared with other NSAIDs for reducing the survival of human prostate cancer cells. Cancer Chemother Pharmacol 50 4: 277–284
Attiga FA, Fernandez PM, Weeraratna AT, Manyak MJ, Patierno SR (2000) Inhibitors of prostaglandin synthesis inhibit human prostate tumor cell invasiveness and reduce the release of matrix metalloproteinases. Cancer Res 60 (16): 4629–4637
Bosetti C, Talamini R, Negri E, Franceschi S, Montella M, La Vecchia C (2006) Aspirin and the risk of prostate cancer. Eur J Cancer Prev 15 (1): 43–45
Brasky TM, Velicer CM, Kristal AR, Peters U, Potter JD, White E (2010) Nonsteroidal anti-inflammatory drugs and prostate cancer risk in the VITamins And Lifestyle (VITAL) cohort. Cancer Epidemiol Biomarkers Prev 19 (12): 3185–3188
Campbell CL, Smyth S, Montalescot G, Steinhubl SR (2007) Aspirin dose for the prevention of cardiovascular disease: a systematic review. JAMA 297 (18): 2018–2024
Cheng I, Liu X, Plummer SJ, Krumroy LM, Casey G, Witte JS (2007) COX2 genetic variation, NSAIDs, and advanced prostate cancer risk. Br J Cancer 97 (4): 557–561
Cheng I, Plummer SJ, Jorgenson E, Liu X, Rybicki BA, Casey G, Witte JS (2008) 8q24 and prostate cancer: association with advanced disease and meta-analysis. Eur J Hum Genet 16 (4): 496–505
Chu LW, Reichardt JK, Hsing AW (2008) Androgens and the molecular epidemiology of prostate cancer. Curr Opin Endocrinol Diabetes Obes 15 (3): 261–270
Dasgupta K, Di Cesar D, Ghosn J, Rajan R, Mahmud S, Rahme E (2006) Association between nonsteroidal anti-inflammatory drugs and prostate cancer occurrence. Cancer J 12 (2): 130–135
De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Tubaro A (2011) The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol 60 (1): 106–117
Dhillon PK, Kenfield SA, Stampfer MJ, Giovannucci EL (2011) Long-term aspirin use and the risk of total, high-grade, regionally advanced and lethal prostate cancer in a prospective cohort of health professionals, 1988–2006. Int J Cancer 128 (10): 2444–2452
Fleming ID, American Joint Committee on Cancer, American Cancer Society, American College of Surgeons (1997) AJCC Cancer Staging Manual 5th edn Lippincott-Raven: Philadelphia
Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Caan BJ (2007) Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidemiol Biomarkers Prev 16 (11): 2218–2225
Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH (2003) A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer 88 (5): 684–688
Garcia Rodriguez LA, Gonzalez-Perez A (2004) Inverse association between nonsteroidal anti-inflammatory drugs and prostate cancer. Cancer Epidemiol Biomarkers Prev 13 (4): 649–653
Gohagan JK, Prorok PC, Hayes RB, Kramer BS (2000) The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 21 (6 Suppl): 251S–272S
Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H (2000) Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate 42 (1): 73–78
Habel LA, Zhao W, Stanford JL (2002) Daily aspirin use and prostate cancer risk in a large, multiracial cohort in the US. Cancer Causes Control 13 (5): 427–434
Hsing AW, Chokkalingam AP (2006) Prostate cancer epidemiology. Front Biosci 11: 1388–1413
Hsu AL, Ching TT, Wang DS, Song X, Rangnekar VM, Chen CS (2000) The cyclooxygenase-2 inhibitor celecoxib induces apoptosis by blocking Akt activation in human prostate cancer cells independently of Bcl-2. J Biol Chem 275 (15): 11397–11403
Jacobs EJ, Newton CC, Stevens VL, Gapstur SM (2011) A large cohort study of long-term acetaminophen use and prostate cancer incidence. Cancer Epidemiol Biomarkers Prev 20 (7): 1322–1328
Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE (2007) Cholesterol-lowering drugs and advanced prostate cancer incidence in a large US cohort. Cancer Epidemiol Biomarkers Prev 16 (11): 2213–2217
Jacobs EJ, Rodriguez C, Mondul AM, Connell CJ, Henley SJ, Calle EE, Thun MJ (2005) A large cohort study of aspirin and other nonsteroidal anti-inflammatory drugs and prostate cancer incidence. J Natl Cancer Inst 97 (13): 975–980
Jacobs EJ, Thun MJ, Bain EB, Rodriguez C, Henley SJ, Calle EE (2007) A large cohort study of long-term daily use of adult-strength aspirin and cancer incidence. J Natl Cancer Inst 99 (8): 608–615
Kamijo T, Sato T, Nagatomi Y, Kitamura T (2001) Induction of apoptosis by cyclooxygenase-2 inhibitors in prostate cancer cell lines. Int J Urol 8 (7): S35–S39
Korn EL, Graubard BI, Midthune D (1997) Time-to-event analysis of longitudinal follow-up of a survey: choice of the time-scale. Am J Epidemiol 145 (1): 72–80
Lee LM, Pan CC, Cheng CJ, Chi CW, Liu TY (2001) Expression of cyclooxygenase-2 in prostate adenocarcinoma and benign prostatic hyperplasia. Anticancer Res 21 (2B): 1291–1294
Leitzmann MF, Ahn J, Albanes D, Hsing AW, Schatzkin A, Chang SC, Andriole GL (2008) Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes Control 19 (10): 1267–1276
Leitzmann MF, Stampfer MJ, Ma J, Chan JM, Colditz GA, Willett WC, Giovannucci E (2002) Aspirin use in relation to risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 11 (10 Pt 1): 1108–1111
Liu X, Plummer SJ, Nock NL, Casey G, Witte JS (2006) Nonsteroidal antiinflammatory drugs and decreased risk of advanced prostate cancer: modification by lymphotoxin alpha. Am J Epidemiol 164 (10): 984–989
Liu XH, Kirschenbaum A, Yao S, Lee R, Holland JF, Levine AC (2000) Inhibition of cyclooxygenase-2 suppresses angiogenesis and the growth of prostate cancer in vivo. J Urol 164 (3 Pt 1): 820–825
Liu XH, Yao S, Kirschenbaum A, Levine AC (1998) NS398, a selective cyclooxygenase-2 inhibitor, induces apoptosis and down-regulates bcl-2 expression in LNCaP cells. Cancer Res 58 (19): 4245–4249
Lloyd FP, Slivova V, Valachovicova T, Sliva D (2003) Aspirin inhibits highly invasive prostate cancer cells. Int J Oncol 23 (5): 1277–1283
Lucia MS, Torkko KC (2004) Inflammation as a target for prostate cancer chemoprevention: pathological and laboratory rationale. J Urol 1712 (Pt 2): S30–S34
Madaan S, Abel PD, Chaudhary KS, Hewitt R, Stott MA, Stamp GW, Lalani EN (2000) Cytoplasmic induction and over-expression of cyclooxygenase-2 in human prostate cancer: implications for prevention and treatment. BJU Int 86 (6): 736–741
Mahmud S, Franco E, Aprikian A (2004) Prostate cancer and use of nonsteroidal anti-inflammatory drugs: systematic review and meta-analysis. Br J Cancer 90 (1): 93–99
Mahmud SM, Franco EL, Aprikian AG (2010) Use of nonsteroidal anti-inflammatory drugs and prostate cancer risk: a meta-analysis. Int J Cancer 127 (7): 1680–1691
Mahmud SM, Franco EL, Turner D, Platt RW, Beck P, Skarsgard D, Aprikian AG (2011) Use of non-steroidal anti-inflammatory drugs and prostate cancer risk: a population-based nested case-control study. PLoS One 6 (1): e16412
Mahmud SM, Tanguay S, Begin LR, Franco EL, Aprikian AG (2006) Non-steroidal anti-inflammatory drug use and prostate cancer in a high-risk population. Eur J Cancer Prev 15 (2): 158–164
Murad AS, Down L, Davey Smith G, Donovan JL, Athene Lane J, Hamdy FC, Martin RM (2011) Associations of aspirin, nonsteroidal anti-inflammatory drug and paracetamol use with PSA-detected prostate cancer: findings from a large, population-based, case-control study (the ProtecT study). Int J Cancer 128 (6): 1442–1448
Nelson JE, Harris RE (2000) Inverse association of prostate cancer and non-steroidal anti-inflammatory drugs (NSAIDs): results of a case-control study. Oncol Rep 7 (1): 169–170
Norrish AE, Jackson RT, McRae CU (1998) Non-steroidal anti-inflammatory drugs and prostate cancer progression. Int J Cancer 77 (4): 511–515
O’Neill GP, Ford-Hutchinson AW (1993) Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett 330 (2): 156–160
Paganini-Hill A, Chao A, Ross RK, Henderson BE (1989) Aspirin use and chronic diseases: a cohort study of the elderly. BMJ 299 (6710): 1247–1250
Perron L, Bairati I, Moore L, Meyer F (2003) Dosage, duration and timing of nonsteroidal antiinflammatory drug use and risk of prostate cancer. Int J Cancer 106 (3): 409–415
Platz EA, De Marzo AM (2004) Epidemiology of inflammation and prostate cancer. J Urol 171 (2 Pt 2): S36–S40
Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E (2006) Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst 98 (24): 1819–1825
Platz EA, Rohrmann S, Pearson JD, Corrada MM, Watson DJ, De Marzo AM, Carter HB (2005) Nonsteroidal anti-inflammatory drugs and risk of prostate cancer in the Baltimore Longitudinal Study of Aging. Cancer Epidemiol Biomarkers Prev 14 (2): 390–396
Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Gohagan JK (2000) Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 21 (6 Suppl): 273S–309S
Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, Furberg CD (1999) Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc 47 (6): 749–754
Roberts RO, Jacobson DJ, Girman CJ, Rhodes T, Lieber MM, Jacobsen SJ (2002) A population-based study of daily nonsteroidal anti-inflammatory drug use and prostate cancer. Mayo Clin Proc 77 (3): 219–225
Salinas CA, Kwon EM, FitzGerald LM, Feng Z, Nelson PS, Ostrander EA, Stanford JL (2010) Use of aspirin and other nonsteroidal antiinflammatory medications in relation to prostate cancer risk. Am J Epidemiol 172 (5): 578–590
Schreinemachers DM, Everson RB (1994) Aspirin use and lung, colon, and breast cancer incidence in a prospective study. Epidemiology 5 (2): 138–146
Siegel R, Naishadham D, Jemal A (2012) Cancer statistics, 2012. CA Cancer J Clin 62 (1): 10–29
Smith WL, DeWitt DL, Garavito RM (2000) Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem 69: 145–182
Soni A (2007) Aspirin Use among the Adult US Noninstitutionalized Population, with and without Indicators of Heart Disease, 2005. Statistical Brief #179 July from http://www.meps.ahrq.gov/mepsweb/data_files/publications/st179/stat179.pdf
Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Baron JA (2003) Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer 88 (11): 1687–1692
Stock D, Groome PA, Siemens DR (2008) Inflammation and prostate cancer: a future target for prevention and therapy? Urol Clin North Am 35 (1): 117–130, vii
Taylor ML, Wells BJ, Smolak MJ (2008) Statins and cancer: a meta-analysis of case-control studies. Eur J Cancer Prev 17 (3): 259–268
Uotila P, Valve E, Martikainen P, Nevalainen M, Nurmi M, Harkonen P (2001) Increased expression of cyclooxygenase-2 and nitric oxide synthase-2 in human prostate cancer. Urol Res 29 (1): 23–28
Weiss NS (2003) Adjusting for screening history in epidemiologic studies of cancer: why, when, and how to do it. Am J Epidemiol 157 (11): 957–961
Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124 (1): 17–27
Witte JS (2007) Multiple prostate cancer risk variants on 8q24. Nat Genet 39 (5): 579–580
Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Isaacs WB (2001) Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res 61 (24): 8617–8623
Acknowledgements
We thank Drs Philip Prorok and Christine Berg at the Division of Cancer Prevention, National Cancer Institute, the Screening Centre investigators and staff of the PLCO Cancer Screening Trial, Tom Riley and staff (Information Management Services, Inc.), and Barbara O’Brien and staff (Westat, Inc.) for their contributions to making this study possible. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License.
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Shebl, F., Sakoda, L., Black, A. et al. Aspirin but not ibuprofen use is associated with reduced risk of prostate cancer: a PLCO Study. Br J Cancer 107, 207–214 (2012). https://doi.org/10.1038/bjc.2012.227
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bjc.2012.227
Keywords
This article is cited by
-
Effect of aspirin on incidence, recurrence, and mortality in prostate cancer patients: integrating evidence from randomized controlled trials and real-world studies
European Journal of Clinical Pharmacology (2023)
-
Associations of low-dose aspirin or other NSAID use with prostate cancer risk in the Danish Diet, Cancer and Health Study
Cancer Causes & Control (2020)
-
Associations between aspirin use and the risk of cancers: a meta-analysis of observational studies
BMC Cancer (2018)
-
Germline BRCA mutation in male carriers—ripe for precision oncology?
Prostate Cancer and Prostatic Diseases (2018)
-
Aspirin and NSAID use in association with molecular subtypes of prostate cancer defined by TMPRSS2:ERG fusion status
Prostate Cancer and Prostatic Diseases (2016)