Abstract
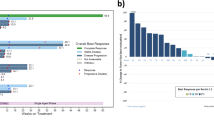
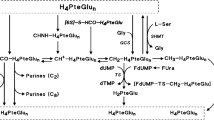
Twenty-nine patients with adenocarcinomas of gastrointestinal or unknown primary, and three with advanced neuroendocrine tumours, were entered into a study of bolus plus infusional 5-fluorouracil (FUra) modulated with high-dose leucovorin (LV) and recombinant interferon alpha 2a (IFN-alpha). Successive cohorts of > or = 4 patients received IFN-alpha at 1.5, 3, 4.5, 6 and 9 MU on alternate days throughout the treatment period. The FUra/LV regimen consisted of: LV 200 mg m-2 i.v. infusion over 2 h, FUra 400 mg m-2 i.v. bolus then FUra 400 mg m-2 i.v. infusion over 22 h, all repeated on day 2, on a 14-day cycle. FUra was given at 75% dose for the first course, increasing (in the absence of WHO grade > or = 2 toxicity) to 87.5% for the second and 100% for subsequent courses up to a maximum of 12. The maximum tolerated dose (MTD) of IFN-alpha was 6 MU on alternate days, with 7/8 patients at 9 MU requiring dose reductions. At 6 MU IFN-alpha, the MTD of FUra was not exceeded at 100% (i.e. 400 mg m-2 bolus and infusion, days 1 and 2), and FUra-related toxicities (mucosal, haematological, dermatological) were extremely mild. Twenty-nine patients were assessable for tumour response, among whom WHO criteria partial responses were seen in 7/14 with colorectal, 1/4 with gastric, 0/1 with pancreatic, 1/3 with neuroendocrine and 3/6 with unknown primaries. Median response duration was 51 weeks. Minor responses and stable disease were seen in a further six patients. Median survival of patients with advanced adenocarcinomas was 9 months, with 33% surviving beyond 18 months. This schedule offers a safe way of co-administering FUra, LV and IFN-alpha. The addition of IFN-alpha, while causing significant independent toxicity, does not significantly increase the dose-limiting mucosal toxicities of FUra/LV. Further investigation is required to determine the contribution of IFN-alpha to the anti-tumour activity of the combination.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
$259.00 per year
only $10.79 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Seymour, M., Johnson, P., Hall, M. et al. Double modulation of 5-fluorouracil with interferon α2a and high-dose leucovorin: a phase I and II study. Br J Cancer 70, 719–723 (1994). https://doi.org/10.1038/bjc.1994.382
Issue Date:
DOI: https://doi.org/10.1038/bjc.1994.382