Key Points
-
Laser use in implantology has been historically controversial.
-
The development of a range of laser wavelengths has shown the adjunctive use of lasers to be more beneficial, both in the healthy and diseased implant case.
-
Laser use in endodontics has advocated benefits in all stages of treatment. However, some are based on anecdote or innovation.
-
The greater investigation into all wavelengths has centred on the anti-bacterial action of laser light energy.
Key Points
Lasers in dentistry
-
1
Introduction, history of lasers and laser light production
-
2
Laser-tissue interaction
-
3
Low-level laser use in dentistry
-
4
Lasers and soft tissue: 'loose' soft tissue surgery
-
5
Lasers and soft tissue: 'fixed' soft tissue surgery
-
6
Lasers and soft tissue: periodontal therapy
-
7
Surgical laser use in implantology and endodontics
-
8
Surgical lasers and hard dental tissue
-
9
Laser regulation and safety in general dental practice
Abstract
The use of surgical lasers has been advocated to aid in the placement and second stage recovery of dental implants, together with soft tissue contouring. In addition, laser use has been suggested as an aid in decontamination of the implant surface in cases of peri-implantitis. In endodontics, the association of laser energy with dentine hypersensitivity, bacteriocidal action and pulp-capping, has led to a growing number of reports as to its beneficial use, together with claims of morphological changes in the canal wall, to enhance endodontic treatment success.
Similar content being viewed by others
The use of lasers in implantology
Surgical lasers can be used in a variety of ways with regard to implantology, ranging from placement, second stage recovery and gingival management, through to the treatment of peri-implantitis. Within this range of usage, dependant on wavelength employed, exists the ablation of target tissue and the ability to reduce bacterial contamination.
Whilst there is a general acceptance that lasers are capable of accurate cutting of materials and tissue, there is no evidence-based advocacy as to the use of any laser wavelength in producing a fully-prepared osteotomy site for the placement of root-form dental implants. However, there are anecdotal reports of the use of erbium YAG and erbium YSGG lasers to establish a controlled incision of overlying gingival tissue and to initiate a breach of the cortical bone plate, prior to the use of conventional implant drills. Such techniques, although intrinsically correctly based on predictable laser-tissue interaction, run the risk of scepticism amongst practitioners more allied to a conventional surgical approach to implant placement.
The fundamental controversy?
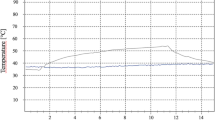
With all other predisposing factors addressed, the fundamental key to success in implant placement is the apposition of normal healing bone onto the implant surface. The preparation of the osteotomy site demands a technique whereby the local temperature does not exceed 47°C.1 Inasmuch as the prime interaction in laser use results in the conversion of incident electromagnetic energy into heat energy, any therapeutic use of lasers in implant dentistry must address this fact. Added to this, once in place, the possibility of implant surface damage arising from incident laser light must be avoided.
The first dental laser, the Nd:YAG (1,064 nm) offered advantages of soft tissue ablation, haemostasis and bacterial control. However, the free-running pulsed emission mode can give rise to peak power values per pulse of >1,000 Watts. Research into the use of this laser as an adjunctive to implantology, drew conclusions that the penetrating and high peak heat energy effects produced during soft tissue and peri-implant treatment, caused damage to both the implant surface and surrounding bone.2,3,4 This led to a general deprecation of laser use in connection with implants, which remained for several years.
With the further development of other laser wavelengths, investigations were carried out to establish whether these newer lasers would cause damage. The general parameters would include the emission mode of the laser (continuous wave (CW), gated pulsed (GP) or free-running pulsed (FRP)), the nature of the target tissue and type of laser-tissue interaction. Other investigations centred around the material used in implant manufacture, its reflectivity, whether the titanium was coated and, generally, the conductive effects of heat through the implant into surrounding bone. Of prime concern is the potential damage to the implant surface and the escalation of heat effects beyond the 47°C threshold in adjacent bone.
Titanium as a metal exhibits reflectivity to incident light energy. With regard to the wavelengths of current lasers, the reflectivity is lowest in the range 780-900 nm, rising as the wavelength increases towards 10,600 nm (CO2 laser emission).5 This would suggest that shorter wavelengths are most damaging, as the low reflectivity would allow greater heat effects to build up, and is in keeping with studies carried out with the Nd:YAG laser. However, there is evidence to suggest that the diode wavelength group, delivered in low power CW values (1-2 Watts average power), cause minimal damage to the implant2 or surrounding bone.6,7 This is explained by the fact that the Nd:YAG, Er,Cr:YSGG and Er:YAG emission modes (FRP), result in high peak power values and heat production (>several hundred °C). Despite the damaging effects of carbon dioxide laser light on bone, several studies have borne out the high reflectivity of titanium to this wavelength, in reporting low thermal effects on the metal surface8,9,10,11,12,13,14 and non-damaging effects on the metal composition.16
Soft tissue management associated with implants
Based on laser-tissue interaction characteristics, all laser wavelengths are suitable for the second stage recovery of implants, provided care is exercised to avoid contact with the implant body (Figs 1, 2, 3, 4, 5, 6, 7, 8). The ablation of soft tissue leads to precise and predictable healing and often this procedure can be carried out using topical anaesthesia.
Suggested energy levels of one to two Watts (CW diode), 150 mJ/15 pps (Nd:YAG), 200-250 mJ/10 pps (erbium group) and one to two Watts (CO2), appear to be appropriate in removing gingival tissue overlying the implant cover screw. The prime advantages of laser use in this procedure would be haemostasis, facilitating easier visual access to the cover screw, production of a protective coagulum as an aid to healing and patient comfort during and after treatment.
Minor surgical correction of the gingival margin can be carried out, to assist adequate implant exposure or to establish the correct emergence profile of the trans-mucosal element (Figs 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19).
As with gingivoplasty around natural teeth, a near-excision approach can be adopted with final detachment of the discard with a sharp curette; alternatively, laboratory-made acrylic copings can be fitted.
Laser use in peri-implantitis
As with conventional treatment approaches, assessment must be made as to the causative factors associated with the condition (infection, occlusion, implant overloading and other local, systemic and life-style factors), and whether the implant is essentially saveable.
Peri-implantitis is recognised as a rapidly progressive failure of osseo-integration,17 in which the production of bacterial toxins precipitates inflammatory change and bone loss.18 The development of peri-implantitis is not restricted to any one type of implant design or construction19,20 and is cited as one of the greater causes of implant loss.21,22 Inasmuch as mechanical debridement together with chemical decontamination (eg chlorhexidine digluconate, citric acid) of the exposed implant surface, with or without site-specific antibiotics, has proved somewhat effective, the possibility to remove bacterial colonisation with an appropriate laser wavelength might well be seen as an added benefit.23,24,25,26,27,28,29,30,31,32 In spite of the risks inherent in using a micro-second pulsed laser, studies by Kreisler et al., using an Er:YAG laser (60-120 mJ/10 pps – 0.6-1.2 W), and Miller, using an Er,Cr:YSGG laser with similar energy parameters, found bacterial kills >99%, without reported damaging effects on the implant surface.33,34
Meticulous attention must be given to curettage of granulation tissue; a laser wavelength that is non-injurious to bone (eg erbium group plus water) can be used to remove this tissue, although careful use of a diode laser, avoiding heat effects (a water spray can be used) and restricting its use to fragmentation and ablation, can be employed. The ability of laser energy in bacterial decontamination appears to place its use above that of other modalities (Figs 20, 21, 22, 23).
However, there is less evidence of beneficial use where the implant is coated with a ceramic or hydroxyapatite; this may be mostly due to the micro-complex surface irregularities, which have been shown to harbour bacteria and foreign ions in a failing situation.35,36
Lasers and endodontics
All current dental laser wavelengths have been used in a wide range of endodontic treatments, either to aid the preparation stages or obturation techniques of root canal therapy or to alleviate low-grade pulpal injury.37,38
The areas of endodontics where laser use has been investigated include the following:
-
Direct pulp capping
-
Removal of pulpal tissue
-
Access/shaping of canal walls and morphological changes in structure
-
Bacterial decontamination
-
Sealing with or removal of gutta percha obturation material
-
Root dentine (cervical) desensitisation.
As with laser use in the debridement of the periodontal pocket, it should be remembered that non-visual access places a potential limit on the control exercised by the operator in using laser energy within the root canal. In addition, laser use should be adjunctive to good clinical practice if benefits are to be maximised. Wherever laser use is indicated, it is recommended that this should be evidence-based and, if deemed appropriate, complementary to all other treatment measures that might be considered. Table 1 lists the procedures that have been advocated and investigated in the field of endodontics and the current commercially available laser wavelengths that are applicable.
Pulp capping and pulpotomy
The consideration for pulp capping and/or pulpotomy using a laser should complement contemporary protocols for such action. Vital pulp exposure (arising from caries or trauma) and subsequent local action, leading to preservation of vital tissue, is contentious in the permanent dentition and success rates are low. It is suggested that permanent teeth with open apices or deciduous teeth offer better chances of pulp reparation.39,40 However, the use of laser energy to aid haemostasis and remove bacterial contamination in order that a reparative dentine bridge could form can offer increased chances of a successful resolution.41,42,43
In a study of 83 patients with 93 teeth treated through a pulp capping procedure, Santucci reported survival rates over 54 months of 43% in teeth treated with calcium hydroxide/resin cement, as opposed to 90% in those teeth treated with Nd:YAG laser and a similar capping cement.44 Moritz et al. (260 teeth treated, 130 study/130 control), reported a tooth vitality two-year survival rate of 93% (control 66%), using a super-pulsed CO2 laser under similar conditions.45
Laser technique involving the exposed vital pulp should be carried out under rubber dam, to prevent contamination with salivary bacteria. Minimal energy levels (1-2 W average power) per wavelength should be employed to provide haemostasis and sterilise the cut surface. A calcium hydroxide dressing should be applied directly, prior to completion of cavity restoration.
Access/shaping of canal walls and morphological changes in structure
The accepted interaction of the Er:YAG and Er,Cr:YSGG lasers with dental hard tissue makes these wavelengths ideal for removal of dentine overlying the pulp chamber.46 The benefit of non-tactile stimulation can aid this procedure in teeth that are tender to percussion and where anaesthesia is incomplete or insufficient.
Within the confines of the root canal, the use of laser wavelengths without water cooling can lead to a potential high rise in temperature. Risks associated include melting/cracking of dentine walls and trans-apical irradiation of the tooth socket.47 With short infrared and CO2 lasers, if benefit is to be obtained, power levels of 0.75-1.5 W should be considered maximal. With water-assisted erbium lasers, power values of 150-250 mJ/4-8 pps are considered suitable, but it is essential to allow water to reach the ablation site, in order to prevent over-heating and cavitation of canal walls.48,49,50
In order to address the end-on emission of laser light from the delivery system, modified intra-canal instruments have been developed51,52 and pre-trial experimental devices to produce non-axial laser light propagation along optic fibres have been investigated (Figs 24, 25, 26).
With mechanical canal preparation, a smear layer is often produced, which can harbour bacteria. Most laser wavelengths will remove the smear layer and can be used in conjunction with irrigants and chelating agents such as NaOCl or EDTA. The Nd:YAG laser has been extensively investigated, but many reports have been made regarding melting and carbonisation.53,54,55,56 It is considered that the erbium group of laser wavelengths is best placed to achieve this, without causing damaging temperature rise.57
Bacterial decontamination
Peri-radicular lesions are diseases either primarily or secondarily caused by micro-organisms. Conventional treatments suggest the combination of mechanical debridement and chemical anti-bacterial agents.58,59
As discussed previously, the anti-bacterial action of laser light is a major benefit of this treatment modality, although in complex canal systems, the use of NaOCl or H2O2 has been shown to be more effective.60,61,62 The fine (200-320 μm) diameters of quartz optic fibres associated with diode and Nd:YAG lasers has enabled these wavelengths to be easily used in bacterial decontamination of the root canal (Figs 27, 28, 29, 30, 31, 32, 33, 34, 35).63,64,65
Of the current lasers available, the CO2 wavelength would appear least successful in effecting bacterial decontamination66 and the effectiveness of laser use appears to depend on fluence values and direct access.67 In addition, some concern has been expressed that the plume produced during laser action might allow bacterial contamination to spread.68,69 As with laser bacterial action in other clinical sites, sub-ablative energy levels should be employed for all wavelengths.
Comparative studies on two common bacterial pathogens, Escherichia coli and E. faecalis have shown that the more complex cell wall of the latter can reduce the effectiveness of laser action. One study by Schoop et al. concluded that diode 810 nm and erbium YAG were better placed to ablate significant numbers of E. faecalis organisms.70 Curiously, in another study by Jha investigating the Er,Cr:YSGG laser, no beneficial bacteriocidal effect could be demonstrated, with either lasers or rotary instrumentation.71 As was reported in the paper on LLLT (BDJ 2007; 202: 131–138), some studies have been carried out, both in vitro and in vivo, into the use of photo-activated disinfection in eliminating intra-canal pathogenic species.72,73,74 In common with studies on many areas of bacterial populations in dentistry, inclusion/exclusion criteria remain significant in determining outcome and can make direct comparison difficult.
Sealing with or removal of gutta percha obturation material
A number of studies have been carried out to establish the usefulness of lasers in the softening and obturation of gutta percha in the root canal (Fig. 36).75,76,77,78,79 However, the development of thermoplastic materials and instruments for such purposes has rendered such application comparatively time consuming and expensive.47
Dentine hypersensitivity
In the absence of any other aetiological factors, 'true' dentine hypersensitivity can be due to gingival recession or toothbrush abrasion and may cause pulpal stimulation through dynamic changes in the intra-tubular proteinaceous fluid.80 Laser–mediated treatment of exposed dentine has been either to address the patency of tubular openings, causing closure of tubule openings to a depth of several microns, or to coagulate the tubular contents.81,82,83 Kimura, in a review of the literature from 1985-2000, ranged the effectiveness of lasers in the treatment of dentine hypersensitivity from 5-100%, dependant upon wavelength and fluence.84 The most commonly explored lasers are the low-level diode (HeNe 633 nm, GaAlAs 810 nm) group and moderate power diode and Nd:YAG.85,86,87,88,89,90 Of these, the use of the Nd:YAG wavelength appears to be more successful. The effectiveness of the low-level group has been proposed through a biostimulatory effect and the higher powered lasers through heat-welding of tubule openings. The erbium group are thought to cause coagulation of tubular contents. Notwithstanding, some studies have called the success of laser use in treating sensitivity into question.91
Energy levels when using hard lasers must be sufficiently low in order to avoid pulpal damage (shorter wavelengths), or tissue ablation (longer wavelengths) and should be of an order of 0.3-0.5 W average power.
Conclusion
The use of lasers in implantology and endodontics has prompted controversy, due either to the essential photothermal action of high powered lasers and its potential for collateral thermal damage, or to the risks associated with 'blind' techniques. The considerable number of investigations carried out into the many permutations of laser wavelengths and target sites has allowed a refinement of criteria and a balanced approach to the claimed success, or otherwise, of laser use. Anecdotal claims as to the effectiveness of this modality continue to drive the need for critical evaluation.
References
Eriksson A, Albrektsson T . Temperature threshold levels for heat-induced bone tissue injury. A vital microscoping study in the rabbit. J Prosthet Dent 1983; 50: 101–107.
Romanos G E, Everts H, Nentwig G H . Effects of diode and Nd:YAG laser irradiation on titanium discs: a scanning electron microscope examination. J Periodontol 2000; 71: 810–815.
Block C, Mayo J, Evans G . Effects of the Nd:YAG dental laser on plasma-sprayed and hydroxyapatite coated titanium dental implants: surface alterations and attempted sterilization. Int J Oral Maxillofac Implants 1992; 7: 441–449.
Kreisler M, Götz H, Duschner H, d'Hoedt B. Effect of the Nd:YAG, Ho:YAG, Er:YAG, CO2 and GaAlAs laser irradiation on surface properties of endosseous dental implants. Med Laser Appl 2001; 16: 152.
Rechmann P, Sadegh H, Goldin D, Hennig T . Surface morphology of implants after laser irradiation. Dtsch Zahnärztl Z 2000; 55: 371–376 [in German].
Kreisler M, Al Haj H, d'Hoedt B . Temperature changes induced by 809-nm GaAlAs laser at the implant-bone interface during simulated surface decontamination. Clin Oral Implants Res 2003; 14: 91–96.
Kreisler M, Schoof J, Langnau E, Al Haj H, d'Hoedt B . Temperature elevations in endosseous dental implants and the peri-implant bone during diode-laser-assisted surface decontamination. Proc SPIE 2002; 4610: 21–30.
Deppe H, Greim H, Brill T, Wagenpfeil S . Titanium deposition after peri-implant care with the carbon dioxide laser. Int J Oral Maxillofac Implants 2002; 17: 707–714.
Swift J, Jenny J, Hargreaves K . Heat generation in hydroxyapatite-coated implants as a result of CO2 laser application. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1995; 79: 410–415.
Oyster D, Parker W, Gher M . CO2 lasers and temperature changes of titanium implants. J Periodontol 1995; 66: 1017–1024.
Ganz C . Evaluation of the safety of the carbon dioxide laser used in conjunction with root form implants. A pilot study. J Prosthet Dent 1994; 71: 27–30.
Barak S, Horowitz I, Katz J, Oelgiesser D . Thermal changes in endosseous root-form implants as a result of CO2 laser application: an in vitro and in vivo study. Int J Oral Maxillofac Implants 1998; 13: 666–671.
Shibli J, Theodoro L, Haypek P, Garcia V, Marcantonio E . The effect of CO2 laser irradiation on failed implant surfaces. Implant Dent 2004; 13: 342–351.
Mouhyi J, Sennerby L, Nammour S, Guillaume P, Van Reck J . Temperature increases during surface decontamination of titanium implants using CO2 laser. Clin Oral Implant Res 1999; 10: 54–61.
Wooten C, Sullivan S, Surpure S . Heat generation by superpulsed CO2 lasers on plasma-sprayed titanium implants: an in vitro study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 1999; 88: 544–548.
Mouhyi J, Sennerby L, Wennerberg A, Louette P, Dourov N, van Reck J . Re-establishment of the atomic composition and the oxide structure of contaminated titanium surfaces by means of carbon dioxide laser and hydrogen peroxide: an in vitro study. Clin Implant Dent Relat Res 2000; 2: 190–202.
Mombelli A . Etiology, diagnosis, and treatment considerations in peri-implantitis. Curr Opin Periodontol 1997; 4: 127–136.
Leonhardt A, Renvert S, Dahlen G . Microbial findings at failing implants. Clin Oral Implants Res 1999; 10: 339–345.
Martins M C, Abi-Rached R S, Shibli J A, Araujo M W, Marcantonio E Jr . Experimental peri-implant tissue breakdown around different dental implant surfaces: clinical and radiographic evaluation in dogs. Int J Oral Maxillofac Implants 2004; 19: 839–848.
Shibli J A, Martins M C, Lotufo R F, Marcantonio E Jr. Microbiologic and radiographic analysis of ligature-induced peri-implantitis with different dental implant surfaces. Int J Oral Maxillofac Implants 2003; 18: 383–390.
Kourtis S G, Sotiriadou S, Voliotis S, Challas A . Private practice results of dental implants. Part I: survival and evaluation of risk factors – Part II: surgical and prosthetic complications. Implant Dent 2004; 13: 373–385.
Oh T J, Yoon J, Misch C E, Wang H L . The causes of early implant bone loss: myth or science? J Periodontol 2002; 73: 322–333.
Romeo E, Ghisolfi M, Murgolo N, Chiapasco M, Lops D, Vogel G . Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res 2005; 16: 9–18.
Augthun M, Tinschert J, Huber A . In vitro studies on the effect of cleaning methods on different implant surfaces. J Periodontol 1998; 69: 857–864.
Buchter A, Meyer U, Kruse-Losler B, Joos U, Kleinheinz J . Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg 2004; 42: 439–444.
Klinge B, Gustafsson A, Berglundh T . A systematic review of the effect of anti-infective therapy in the treatment of peri-implantitis. J Clin Periodontol 2002; 29 (Suppl 3): 213–225.
Tang Z, Cao C, Sha Y, Lin Y, Wang X . Effects of non-surgical treatment modalities on peri-implantitis. Zhonghua Kou Qiang Yi Xue Za Zhi 2002; 37: 173–175.
Bunetel L, Guerin J, Agnani G et al. In vitro study of the effect of titanium on Porphyromonas gingivalis in the presence of metronidazole and spiramycin. Biomaterials 2001; 22: 3067–3072.
Leonhardt A, Dahlen G, Renvert S . Five-year clinical, microbiological, and radiological outcome following treatment of peri-implantitis in man. J Periodontol 2003; 74: 1415–1422.
Walsh L . The use of lasers in implantology: an overview. J Oral Implantol 1992; 18: 335–340.
Haas R, Dörtbudak O, Mensdorff-Pouilly N, Mailath G . Elimination of bacteria on different implant surfaces through photosensitization and soft laser. Clin Oral Implants Res 1997; 8: 249–254.
Kato T, Kusakari H, Hoshino E . Bactericidal efficacy of carbon dioxide laser against bacteria-contaminated implants and subsequent cellular adhesion to irradiated area. Lasers Surg Med 1998; 23: 299–309.
Kreisler M, Kohnen W, Marinello C et al. Bactericidal effect of the Er:YAG laser on dental implant surfaces: an in vitro study. J Periodontol 2002; 73: 1292–1298.
Miller R J . Treatment of the contaminated implant surface using the Er,Cr:YSGG laser. Implant Dent 2004; 13: 165–170.
Ichikawa T, Hirota K, Kanitani H, Miyake Y, Matsumoto N . In vitro adherence of Streptococcus constellatus to dense hydroxyapatite and titanium. J Oral Rehabil 1998; 25: 125–127.
MacDonald D E, Betts F, Doty S B, Boskey A L . A methodological study for the analysis of apatite-coated dental implants retrieved from humans. Ann Periodontol 2000; 5: 175–184.
Stabholz A, Sahar-Helft S, Moshonov J . Lasers in endodontics. Dent Clin North Am 2004; 48: 809–832.
Kimura Y, Wilder-Smith P, Matsumoto K . Lasers in endodontics: a review. Int Endod J 2000; 33: 173–185.
Shoji S, Nakamura M, Horiuchi H . Histopathological changes in dental pulps irradiated by CO2 laser: a preliminary report on laser pulpotomy. J Endod 1985; 11: 773–780.
Dang J, Wilder-Smith P, Peavy G . Clinical preconditions and treatment modality: effects on pulp surgery outcome. Lasers Surg Med 1998; 22: 25–29.
Liu H, Yan M M, Zhao E Y, Chen L, Liu H W . Preliminary report on the effect of Nd: YAG laser irradiation on canine tooth pulps. Chin J Dent Res 2000; 3(4): 63–65.
Jayawardena J A, Kato J, Moriya K, Takagi Y . Pulpal response to exposure with Er:YAG laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2001; 91: 222–229.
Kimura Y, Yonaga K, Yokoyama K, Watanabe H, Wang X, Matsumoto K . Histopathological changes in dental pulp irradiated by Er:YAG laser: a preliminary report on laser pulpotomy. J Clin Laser Med Surg 2003; 21: 345–350.
Santucci P J . Dycal versus Nd:YAG laser and Vitrebond for direct pulp capping in permanent teeth. J Clin Laser Med Surg 1999; 17: 69–75.
Moritz A, Schoop U, Goharkhay K, Sperr W . Advantages of a pulsed CO2 laser in direct pulp capping: a long-term in vivo study. Lasers Surg Med 1998; 22: 288–293.
Chen W H . Laser root canal therapy. J Indiana Dent Assoc 2002–2003; 81: 20–23.
Anic I, Tachibana H, Matsumoto K, Qi P . Permeability, morphologic and temperature changes of canal dentine walls induced by Nd:YAG, CO2 and argon lasers. Int Endod J 1996; 29: 13–22.
Ebihara A, Majaron B, Liaw L H, Krasieva T B, Wilder-Smith P . Er:YAG laser modification of root canal dentine: influence of pulse duration, repetitive irradiation and water spray. Lasers Med Sci 2002; 17: 198–207.
Yamazaki R, Goya C, Yu D G, Kimura Y, Matsumoto K . Effects of erbium,chromium:YSGG laser irradiation on root canal walls: a scanning electron microscopic and thermographic study. J Endod 2001; 27: 9–12.
Kimura Y, Yonaga K, Yokoyama K, Kinoshita J, Ogata Y, Matsumoto K . Root surface temperature increase during Er:YAG laser irradiation of root canals. J Endod 2002; 28: 76–78.
Shoji S, Hariu H, Horiuchi H . Canal enlargement by Er:YAG laser using a cone-shaped irradiation tip. J Endod 2000; 26: 454–458.
Kesler G, Gal R, Kesler A, Koren R . Histological and scanning electron microscope examination of root canal after preparation with Er:YAG laser microprobe: a preliminary in vitro study. J Clin Laser Med Surg 2002; 20: 269–277.
Kaitsas V, Signore A, Fonzi L, Benedicenti S, Barone M . Effects of Nd: YAG laser irradiation on the root canal wall dentin of human teeth: a SEM study. Bull Group Int Rech Sci Stomatol Odontol 2001; 43: 87–92.
Levy G . Cleaning and shaping the root canal with a Nd:YAG laser beam: a comparative study. J Endod 1992; 18: 123–127.
Miserendino L, Levy G, Rizoiu I . Effects of Nd:YAG laser on the permeability of root canal wall dentin. J Endod 1995; 21: 83–87.
Zhang C, Kimura Y, Matsumoto K, Harashima T, Zhou H . Effects of pulsed Nd:YAG laser irradiation on root canal wall dentin with different laser initiators. J Endod 1998; 24: 352–355.
Matsuoka E, Kimura Y, Matsumoto K . Studies on the removal of debris near the apical seats by Er:YAG and assessment with a fiberscope. J Clin Laser Med Surg 1998; 16: 255–261.
Siqueira Junior J F . Strategies to treat infected root canals. J Calif Dent Assoc 2001; 29: 825–837.
Piccolomini R, D'Arcangelo C, D'Ercole S, Catamo G, Schiaffino G, De Fazio P . Bacteriologic evaluation of the effect of Nd:YAG laser irradiation in experimental infected root canals. J Endod 2002; 28: 276–278.
Schoop U, Moritz A, Kluger W et al. The Er:YAG laser in endodontics: results of an in vitro study. Lasers Surg Med 2002; 30: 360–364.
Perin F M, Franca S C, Silva-Sousa Y T et al. Evaluation of the antimicrobial effect of Er:YAG laser irradiation versus 1% sodium hypochlorite irrigation for root canal disinfection. Aust Endod J 2004; 30: 20–22.
Folwaczny M, Mehl A, Jordan C, Hickel R . Antibacterial effects of pulsed Nd:YAG laser radiation at different energy settings in root canals. J Endod 2002; 28: 24–29.
Rooney J, Midda M, Leeming J . A laboratory investigation of the bactericidal effect of a Nd:YAG laser. Br Dent J 1994; 176: 61–64.
Fegan S, Steiman H . Comparative evaluation of the antibacterial effects of intracanal Nd:YAG laser irradiation: an in vitro study. J Endod 1995; 21: 415–417.
Moritz A, Gutknecht N, Gohrakhay K, Schoop U, Wernisch J, Sperr W . In vitro irradiation of infected root canals with a diode laser: results of microbiologic, infrared spectrometric, and stain penetration examinations. Quintessence Int 1997; 28: 205–209.
Le Goff A, Dautel-Morazin A, Guigand M, Vulcain J M, Bonnaure-Mallet M. An evaluation of the CO2 laser for endodontic disinfection. J Endod 1999; 25: 105–108.
Kreisler M, Kohnen W, Beck M et al. Efficacy of NaOCl/H2O2 irrigation and GaAlAs laser in decontamination of root canals in vitro. Lasers Surg Med 2003; 32: 189–196.
McKinley I, Ludlow M . Hazards of laser smoke during endodontic therapy. J Endod 1994; 20: 558–559.
Hardee M, Miserendino L, Kos W, Walia H . Evaluation of the antibacterial effects of intracanal Nd:YAG laser irradiation. J Endod 1994; 20: 377–380.
Schoop U, Kluger W, Moritz A, Nedjelik N, Georgopoulos A, Sperr W . Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med 2004; 35: 111–116.
Jha D, Guerrero A, Ngo T, Helfer A, Hasselgren G . Inability of laser and rotary instrumentation to eliminate root canal infection. J Am Dent Assoc 2006; 137: 67–70.
Williams J A, Pearson G J, John Colles M . Antibacterial action of photoactivated disinfection {PAD} used on endodontic bacteria in planktonic suspension and in artificial and human root canals. J Dent 2006; 34: 363–371.
Bonsor S J, Nichol R, Reid T M S, Pearson G J . Microbiological evaluation of photo-activated disinfection in endodontics (an in vivo study). Br Dent J 2006; 200: 337–341.
Lee M T, Bird P S, Walsh L J . Photo-activated disinfection of root canals: a new role for lasers in endodontics. Aust Endod J 2004; 30: 93–98.
Anjo T, Ebihara A, Takeda A, Takashina M, Sunakawa M, Suda H . Removal of two types of root canal filling material using pulsed Nd:YAG laser irradiation. Photomed Laser Surg 2004; 22: 470–476.
Viducic D, Jukic S, Karlovic Z, Bozic Z, Miletic I, Anic I . Removal of gutta-percha from root canals using an Nd:YAG laser. Int Endod J 2003; 36: 670–673.
Carvalho C A, Valera M C, Gown-Soares S, de Paula Eduardo C . Effects of Nd:YAG and Er:YAG lasers on the sealing of root canal fillings. J Clin Laser Med Surg 2002; 20: 215–219.
Maden M, Gorgul G, Tinaz A C . Evaluation of apical leakage of root canals obturated with Nd: YAG laser-softened gutta-percha, System-B, and lateral condensation techniques. J Contemp Dent Pract 2002; 3: 16–26.
Kimura Y, Yonaga K, Yokoyama K, Matsuoka E, Sakai K, Matsumoto K . Apical leakage of obturated canals prepared by Er:YAG laser. J Endod 2001; 27: 567–570.
Absi E G, Addy M, Adams D . Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol 1987; 14: 280–284.
Liu H C, Lin C P, Lan W H . Sealing depth of Nd:YAG laser on human dentinal tubules. J Endod 1997; 23: 691–693.
Schwarz F, Arweiler N, Georg T, Reich E . Desensitizing effects of an Er:YAG laser on hypersensitive dentine. J Clin Periodontol 2002; 29: 211–215.
Zhang C, Matsumoto K, Kimura Y, Harashima T, Takeda F H, Zhou H . Effects of CO2 laser in treatment of cervical dentinal hypersensitivity. J Endod 1998; 24: 595–597.
Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K . Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 2000; 27: 715–721.
Marsilio A L, Rodrigues J R, Borges A B . Effect of the clinical application of the GaAlAs laser in the treatment of dentine hypersensitivity. J Clin Laser Med Surg 2003; 21: 291–296.
Corona S A, Nascimento T N, Catirse A B, Lizarelli R F, Dinelli W, Palma-Dibb R G . Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 2003; 30: 1183–1189.
Walsh L J . The current status of low level laser therapy in dentistry. Part 2. Hard tissue applications. Aust Dent J 1997; 42: 302–306.
Renton-Harper P, Midda M . Nd:YAG laser treatment of dentinal hypersensitivity. Br Dent J 1992; 172: 13–16.
Lan W H, Lee B S, Liu H C, Lin C P . Morphologic study of Nd:YAG laser usage in treatment of dentinal hypersensitivity. J Endod 2004; 30: 131–134.
Ciaramicoli M T, Carvalho R C, Eduardo C P . Treatment of cervical dentin hypersensitivity using neodymium: yttrium-aluminum-garnet laser. Clinical evaluation. Lasers Surg Med 2003; 33: 358–362.
Lier B B, Rosing C K, Aass A M, Gjermo P . Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Periodontol 2002; 29: 501–506.
Author information
Authors and Affiliations
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Parker, S. Surgical laser use in implantology and endodontics. Br Dent J 202, 377–386 (2007). https://doi.org/10.1038/bdj.2007.284
Published:
Issue Date:
DOI: https://doi.org/10.1038/bdj.2007.284
This article is cited by
-
Erbium YAG laser and diode laser applications for the second phase of implant surgery: a comparison of clinical outcomes
Lasers in Dental Science (2019)
-
Different laser wavelengths comparison in the second-stage implant surgery: an ex vivo study
Lasers in Medical Science (2015)
-
Laser list
British Dental Journal (2007)