Abstract
BACKGROUND: Although inhaled nitric oxide has been shown to reduce the use of extracorporeal membrane oxygenation, there is limited data on its effect on long-term outcomes.
OBJECTIVE: The purpose of our study is to report on the 1 year outcome of neonates treated with inhaled nitric oxide compared to a group of neonates who did not receive nitric oxide.
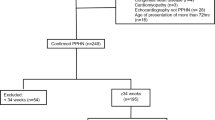
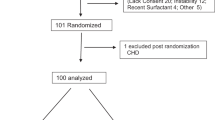
METHODS: We have previously reported on the in-hospital outcomes of 248 neonates who were >34 weeks' gestational age and were randomized to receive low-dose inhaled nitric oxide or placebo. Extracorporeal membrane oxygenation was used in 78 (64%) neonates in the control group and in 48 (38%) neonates in the inhaled nitric oxide group (p=0.001). We now report on the outcome of neonates followed to 1 year of age.
RESULTS: Of the 248 neonates twenty-four (10%) died before 1 year of age. There was no difference in mortality between the two groups (11% in the control group and 9% in the inhaled nitric oxide group). Of the 224 surviving infants, we were able to contact the parents or guardians of 201 (90%) children. There were no intergroup differences in the numbers of patients reported as requiring medications for pulmonary disease (14% in the control group and 14% in the inhaled nitric oxide group) or the need for supplemental oxygen (1% in the control group and 0% in the inhaled nitric oxide group). The number of neonates reported to have an abnormal neurological examination or developmental delay was also similar in both groups (14% in the control group and 19% in the inhaled nitric oxide group).
CONCLUSIONS: The use of low-dose inhaled nitric oxide reduces the use of extracorporeal membrane oxygenation without increasing the incidence of adverse outcomes to 1 year of age.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med 2000;342:469–474.
Kinsella JP, Abman SH . Recent developments in inhaled nitric oxide therapy of the newborn. Curr Opin Pediatr 1999;11:121–125.
The Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in term and near-term infants: neurodevelopmental follow-up of the neonatal inhaled nitric oxide study group (NINOS). J Pediatr 2000;136:611–617.
Davidson D, Barefield ES, Kattwinkel J, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: A randomized, double-masked, placebo-controlled, dose-response, multicenter study. The I-NO/PPHN Study Group. Pediatrics 1998;101:325–334.
Kinsella JP, Truog WE, Walsh WF, et al. Randomized multicenter trial of inhaled nitric oxide and high-frequency oscillatory ventilation in severe persistent pulmonary hypertension of the newborn. J Pediatr 1997;131:55–62.
Roberts Jr. JD, Fineman JR, Morin FC III, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn The Inhaled Nitric Oxide Study Group. N Engl J Med 1997;336:605–610.
Lipkin PH, Davidson D, Spivak L, Straube R, Rhines J, Chang CT . Neurodevelopmental and medical outcomes of persistent pulmonary hypertension in term newborns treated with nitric oxide. J Pediatr 2002; 140:306–310.
Rosenberg AA . Outcome in term infants treated with inhaled nitric oxide. J Pediatr 2002;140:284–287.
Vaucher YE, Dudell GG, Bejar R, Gist K . Predictors of early childhood outcome in candidates for extracorporeal membrane oxygenation. J Pediatr 1996;128:109–117.
Walsh-Sukys MC, Bauer RE, Cornell DJ, Friedman HG, Stork EK, Hack M . Severe respiratory failure in neonates: mortality and morbidity rates and neurodevelopmental outcomes. J Pediatr 1994;125:104–110.
Schwendeman CA, Clark RH, Yoder BA, Null Jr, DM, Gerstmann DR, deLemos RA . Freuency of chronic lung disease in infants with severe respiratory failure treated with high-freuency ventilation and/or extracorporeal membrane oxygenation. Crit Care Med 1992;20:372–377.
Bennett CC, Johnson A, Field DJ, Elbourne D . UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation: follow-up to age 4 years. Lancet 2001;357:1094–1096.
Acknowledgements
This study was funded in part by a grant from INO Therapeutics, Inc., Liberty Corner, NJ, USA. Dr. Reese Clark has acted as a consultant to INO Therapeutics, Inc. regarding submission of data to the US Food & Drug Administration (FDA). He is also the principal investigator for the grant that supports this study. Ms. Jeryl Huckaby has acted as a clinical research associate for INO Therapeutics, Inc. in monitoring this study's case report forms. None of the authors own stock or have any financial interest in INO Therapeutics, Inc.
Author information
Authors and Affiliations
Consortia
Appendix
Appendix
The Collaborative Inhaled Nitric Oxide Research Initiative (CINRGI) included the following institutions and investigators. Akron, OH — J. Butler, K. Wellendorf; Durham, NC — K. Auten; Phoenix, AZ — D. Hall, E. Ramthun; Atlanta, GA — J. Huckaby, L. Jain, B. Roy, I. Seabrook; Washington, DC — M. Keszler, P. Angelus; Charlotte, NC — T. Kueser, L. Brucoli; Columbia, SC — D. Marsh, A. O'Dell; New Orleans, LA — M. McGettigan, B. uinn, G. Matranga; St. Petersburg, FL — A. Napolitano, R. Williams; San Antonio, TX — M. Odom; Orlando, FL — J. Perez, J. Ramos; Chicago, IL — M. Rathi, A. Shukla, T. Gardner; Charleston, SC — M. Southgate, D. Purohit, S. Ballard, M. Nussbaum; Sioux Falls, SD — D. Stevens, R. Klinghagen; Greenville, SC — W. Walker, V. Jenkinson; Nashville, TN — W. Walsh, S. Steele, B. Canter; Lackland AFB, TX — B. Yoder, S. Woodcox.
Rights and permissions
About this article
Cite this article
Clark, R., Huckaby, J., Kueser, T. et al. Low-Dose Nitric Oxide Therapy for Persistent Pulmonary Hypertension: 1-Year Follow-up. J Perinatol 23, 300–303 (2003). https://doi.org/10.1038/sj.jp.7210908
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7210908
This article is cited by
-
Occurrence of hyperoxia during iNO treatment for persistent pulmonary hypertension of the newborn: a cohort study
European Journal of Pediatrics (2024)
-
Worsened short-term clinical outcomes in a cohort of patients with iNO-unresponsive PPHN: a case for improving iNO responsiveness
Journal of Perinatology (2022)
-
Polymorphisms in urea cycle enzyme genes are associated with persistent pulmonary hypertension of the newborn
Pediatric Research (2018)
-
Altered metabolites in newborns with persistent pulmonary hypertension
Pediatric Research (2018)
-
Variations in CRHR1 are associated with persistent pulmonary hypertension of the newborn
Pediatric Research (2012)