Abstract
OBJECTIVE:To evaluate the feasibility of conducting a prospective, randomized trial comparing early high-frequency oscillatory ventilation (HFOV) to synchronized intermittent mandatory ventilation (SIMV) in very low birth weight (VLBW) premature infants. This pilot study evaluated two ventilator management protocols to determine how well they could be implemented in a multicenter clinical trial. Although this pilot study was not powered to detect differences in outcome, we also collected outcome data.
DESIGN:Prospective, multicenter, randomized pilot study.
SETTING:Seven tertiary-level intensive care nurseries with previous experience with both HFOV and flow-triggered SIMV.
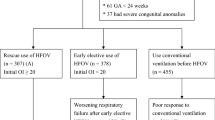
PATIENTS:Fifty infants weighing 501 to 1200 g, less than 4 hours of age, who had received one dose of surfactant and required ventilation with mean airway pressure ≥6 cm H 2O and F IO 2 ≥0.25, and had an anticipated duration of ventilation greater than 24 hours.
INTERVENTIONS:Patients were stratified by birth weight and prenatal steroid status, then randomized to either HFOV or SIMV with tidal volume monitoring. Ventilator management for patients in both study arms was strictly governed by protocols that included optimizing lung inflation and blood gases, weaning strategies, and extubation criteria.
MEASUREMENTS:Data were collected using the tools planned for the larger collaborative study. Protocol compliance was closely monitored, with successive changes in the protocol made as necessary to improve clarity and increase compliance. The incidence of major neonatal adverse outcomes was recorded.
MAIN RESULTS:Data are presented for 24 HFOV and 24 SIMV infants (two infants, twins, were withdrawn from the study at parent's request). Nineteen of the 24 HFOV infants and 20 of the 24 SIMV infants survived to 36 weeks corrected age. Age at final extubation for survivors was 16±16 (mean±SD) days for HFOV infants and 24±24 days for SIMV infants. At 36 weeks corrected age, 14 of the 19 HFOV survivors were extubated and in room air, whereas 5 required supplemental oxygen. In comparison, 6 of the 20 SIMV survivors were extubated and in room air, whereas 14 required supplemental oxygen. Grade III/IV IVH and/or periventricular leukomalacia occurred in 2 HFOV and 2 SIMV patients.
Overall compliance with the ventilator protocols was 82% for the SIMV protocol, and 88% for the HFOV protocol.
CONCLUSIONS:The preliminary outcome data supports conducting the large randomized trial, which began in July of 1998. The protocols for the ventilator management of VLBW infants, both with HFOV and with SIMV were easily implemented and consistently followed, and are presented here.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Fenton AC, Mason E, Clarke M, Field DJ Chronic lung disease following neonatal ventilation: II. Changing incidence in a geographically defined population Pediatr Pulmonol 1996 21 24–7
Durand DJ, Asselin JM In: Polin R, Fox W, editors. Physiology of High Frequency Ventilation. Fetal and Neonatal Physiology WB Saunders, Philadelphia, PA 1998 Chapter 110 pp. 1212–9
Hamilton PP, Onayemi A, Smyth JA, et al Comparison of conventional and high frequency ventilation: oxygenation and lung pathology J Appl Physiol 1983 55 131–8
deLemos RA, Coalson JJ, Gerstmann DR, et al Ventilatory management of infant baboons with hyaline membrane disease: the use of high frequency ventilation Pediatr Res 1987 21 594–602
McCulloch PR, Forkert PG, Froese AB Lung volume maintenance prevents lung injury during high frequency oscillatory ventilation in surfactant deficient rabbits Am Rev Respir Dis 1988 137 1185–92
deLemos RA, Coalson JJ, Meredith KS, et al A comparison of ventilation strategies for the use of high frequency oscillatory ventilation in the treatment of hyaline membrane disease Acta Anaesthesiol Scand 1989 33 102–107
Meredith KS, deLemos RA, Coalson JJ, et al Role of lung injury in the pathogenesis of hyaline membrane disease in premature baboons J Appl Physiol 1989 66 2150–8
Kinsella JP, Gerstmann DR, Clark RH, et al High-frequency oscillatory ventilation versus intermittent mandatory ventilation: early hemodynnamic effects in the premature baboon with hyaline membrane disease Pediatr Res 1991 29 160–6
Jackson JC, Troug WE, Standaert TA, et al Effect of high frequency ventilation on the development of alveolar edema in premature monkeys at risk for hyaline membrane disease Am Rev Respir Dis 1991 143 865–71
HiFi Study Group High-frequency oscillatory ventilation compared with conventional mechanical ventilation in the treatment of respiratory failure in preterm infants N Engl J Med 1989 320 88–93
Keszler M, Donn SM, Bucciarelli RL, et al Multicenter controlled trial comparing high-frequency jet ventilation and conventional mechanical ventilation in newborn infants with pulmonary interstitial emphysema J Pediatr 1991 119 85–93
Clark RH, Gerstmann DR, Null DM, deLemos RA Prospective randomized comparison of high-frequency oscillatory and conventional ventilation in respiratory distress syndrome Pediatrics 1992 89 5–12
HiFO Study Group Randomized study of high-frequency oscillatory ventilation in infants with severe respiratory distress syndrome J Pediatr 1993 122 609–9
Ogawa Y, Miyasaka K, Kawano, et al A multicenter randomized trial of high frequency oscillatory ventilation as compared with conventional mechanical ventilation in preterm infants with respiratory failure Early Hum Dev 1993 32 1–10
Thome U, Kossel H, Lipowsky G, et al Randomized comparison of high-frequency ventilation with high-rate intermittent positive pressure ventilation in preterm infants with respiratory failure J Pediatr 1999 135 39–46
Froese AB, McCulluch PR, Sugiura M, et al Optimizing alveolar expansion prolongs the effectiveness of exogenous surfactant therapy in the adult rabbit Am Rev Respir Dis 1993 148 569–77
Jackson JC, Truog WE, Standaert TA, et al Reduction in lung injury after combined surfactant and high frequency ventilation Am J Respir Crit Care Med 1994 150 534–9
Gerstmann DR, Minton SD, Stoddard RA, et al The Provo multicenter early high-frequency oscillatory ventilation trial: Improved pulmonary and clinical outcome in respiratory distress syndrome Pediatrics 1996 98 1044–57
Keszler M, Modanlou HD, Brudno DS, et al Multicenter controlled clinical trial of high-frequency jet ventilation in preterm infants with uncomplicated respiratory distress syndrome Pediatrics 1997 100 593–9
Bernstein G, Heldt GP, Mannino R Increased and more consistent tidal volumes during synchronized intermittent mandatory ventilation in newborn infants Am J Respir Crit Care Med 1994 150 1444–8
Cleary JP, Bernstein G, Mannino FL, Heldt GP Improved oxygenation during synchronized intermittent mandatory ventilation in neonates with respiratory distress syndrome: a randomized corssover study J Pediatr 1995 126 407–11
Bernstein G, Mannino FL, Heldt GP, et al Randomized multicenter trial comparing synchronized and conventional intermittent mandatory ventilation in neonates J Pediatr 1996 128 453–3
Zubrow AB, Hulman S, Kushner H, Falkner B, Philadelphia Neonatal Blood Pressure Study Group Determinants of blood pressure in infants admitted to neonatal intensive care units: a prospective multicenter study J Perinatol 1995 15 470–9
Gerstmann DR, Fouke JM, Winter DC, et al Proximal, tracheal, and alveolar pressures during high frequency oscillatory ventilation in a normal rabbit model Pediatr Res 1990 28 367–73
Durand DJ, Goodman A, Rey P, et al Theophylline treatment in the extubation of infants weighing less than 1250 grams: a controlled trial Pediatrics 1987 80 684–8
So BH, Tamura M, Mishina J, et al Application of nasal continuous positive airway pressure to early extubation in very low birthweight infants Arch Dis Child 1995 72:F191–3
Higgins RD, Richter SE, Davis JM Nasal continuous positive airway pressure facilitates extubation of very low birth weight neonates Pediatrics 1991 88 999–1003
Ment LR, Oh W, Ehrenkranz RA, et al Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial Pediatrics 1994 93 543–50
Baumer JH International randomized controlled trial of patient triggered ventilation in neonatal respiratory distress syndrome Arch Dis Child Fetal Neonat Ed 2000 82 F5–F10
Clark RH, Slutsky AS, Gerstmann DR Lung protective strategies of ventilation in the neonate: what are they? Pediatrics 2000 105 112–4
The Acute Respiratory Distress Syndrome Network Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome N Engl J Med 2000 342 1301–8
Wada K, Jobe AH, Ikegami M Tidal volume effects on surfactant treatment responses with the initiation of ventilation in preterm lambs J Appl Physiol 1997 84 1054–61
Merrit TA, Palmer D, Bergman DA, Shiono PH Clinical practice guidelines in pediatric and newborn medicine: implications for their use in practice Pediatrics 1997 99 100–14
Kollef MH, Shapiro SD, Silver P, et al A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation Crit Care Med 1997 25 567–74
Acknowledgements
This pilot study could not have been completed without the help of many individuals. The Steering Committee included David J. Durand, MD, Jeanette M. Asselin, RRT MS, Sherry E. Courtney, MD MS, Mark L. Hudak, MD, Thomas E. Wiswell, MD, Craig T. Shoemaker, MD, and Judy L. Aschner, MD. The study sites included: Children's Hospital Oakland, Oakland CA; Wolfson Children's Hospital, University of Florida, Jacksonville, FL; Wake Forest University School of Medicine, Winston-Salem, NC; New Hanover Regional Medical Center, Wilmington, NC; Children's Hospital Orange County, Orange, CA; Lucille Packard Children's Hospital at Stanford, Palo Alto, CA; Kosair Children's Hospital, University of Louisville, Louisville, KY; MeritCare Medical Center, Fargo, ND; Cooper Hospital/University Medical Center, Camden, NJ. The masked ultrasound reviewers included Daniel A. Merton, RDMS and Larry Needleman, MD from Thomas Jefferson University Medical Center, Philadelphia, PA.
Author information
Authors and Affiliations
Additional information
This study was presented in part at the Society for Pediatric Research in May 1998 in New Orleans, LA. This study was supported in part by Abbott Laboratories/Ross Products Division, Hamilton Medical, Ballard Medical Products, SensorMedics, the Society for Pediatric Research, the East Bay Neonatology Foundation, and Cooper Hospital/UMC.
APPENDIX
APPENDIX
The underlying philosophy of the following protocols includes optimizing lung inflation, avoiding both atelectasis and overdistension, tolerating moderate hypercapnia, maintaining oxygen saturation within a narrow range, and aggressively weaning toward extubation. The frequency and magnitude of ventilator changes outlined in the protocol are initial guidelines; larger or more frequent ventilatory changes may be required to maintain infants in the target ranges. It is essential that every effort be made to rapidly return infants to the target ranges if they have “drifted” outside these ranges.
Target Ranges for Blood Gas Values
Because the target ranges are fairly broad, weaning of ventilator settings is strongly encouraged for patients who have P aCO 2 values in the lower end of the target ranges. Likewise, weaning of patients with stable oxygenation is strongly encouraged.
The target ranges for blood gases are as follows:
-
Pulse oximeter saturation 88% to 96%;
-
pH≥7.25.
-
P aCO 2 40 to 55 Torr in patients without PIE, gross air leak, hyperinflation, chronic changes on chest X-ray (CXR);
-
P aCO 2 45 to 65 Torr in patients with PIE, gross air leak, hyperinflation, or chronic changes on CXR;
Target Ranges for Lung Inflation
“Ideal” lung inflation is defined as the top margin of the dome of the right hemidiaphragm located between the bottom of the eighth rib and no more than midway between the ninth and tenth ribs. The major focus will be avoidance of atelectasis and hyperinflation. In addition to overexpansion, small heart and flat diaphragms may also indicate hyperinflation. Patients with PIE or AL will be managed with a low-lung-volume strategy, defined as diaphragm level between the seventh and eighth ribs.
Nontidal Volume Ventilation Strategy (HFOV)
Initial HFOV settings
Initiate HFOV at these settings:
-
Inspiratory time ( Tinsp) 33%;
-
Mean airway pressure ( Paw) at least 2 cm H 2O greater than patient was receiving on conventional ventilation. This apparent increase is because HFOV causes a pressure measurement artifact that results in a Paw reading that is 2 cm H 2O higher at the proximal tip of the ETT than the actual distal Paw;
-
Frequency 10 to 15 Hz;
-
Amplitude (Δ P) adjusted based on physical examination and/or transcutaneous monitoring.
Adjusting HFOV settings to optimize lung inflation
The initial goal on HFOV is to optimize lung inflation. The optimal position of the right hemidiaphragm is between 8 and 9.5 ribs. Diaphragm position is primarily determined by Paw. If top dome of right diaphragm is:
-
Below the 11th rib, decrease Paw by 2 cm H 2O;
-
Between the 10th and 11th rib, decrease Paw by 1 cm H 2O;
-
Between 8 and 9.5 ribs, no change;
-
Above the eighth rib, increase Paw by 1 cm H 2O;
-
Above the seventh rib, increase Paw by 2 cm H 2O;
-
Above the eighth rib, but infant in room air, increasing Paw is optional.
Adjusting HFOV settings based on F I O 2
Assuming acceptable lung inflation, adjust Paw based on F IO 2
-
F IO 2 ≥0.40, increase Paw 1 to 2 cm H 2O;
-
F IO 2 0.30 to 0.39, may increase Paw or make no change, depending on CXR;
-
F IO 2 <0.30, decrease Paw 1 to 2 cm H 2O;
-
If F IO 2 changes by 0.2 within a 6-hour period, repeat a CXR to evaluate lung inflation.
Adjusting HFOV settings based on P a CO 2
Assuming a constant HFOV frequency, P aCO 2 is primarily determined by HFOV amplitude, measured as Δ P.
-
P aCO 2 <30 Torr, decrease Δ P by 20%;
-
P aCO 2 30 to 39 Torr, decrease Δ P by 10%;
-
P aCO 2 40 to 55 Torr, no change in Δ P is necessary unless P aCO 2 has been in this range for >12 hours (see HFOV Weaning);
-
P aCO 2 56 to 65, increase Δ P by 10%;
-
P aCO 2 >65, increase Δ P by 20%;
-
During initial stabilization, repeat arterial blood gas every 20 to 30 minutes until the P aCO 2 is within the target range.
HFOV management of PIE and/or gross air leak with low-lung-volume strategy
-
If the infant has PIE or gross air leak such as pneumothorax or pneumomediastinum, change to a low-lung-volume strategy (diaphragm level between seventh and eighth ribs) accepting whatever F IO 2 is necessary to maintain target blood gases, until the air leak has resolved for ≥24 hours.
-
If the infant has unilateral PIE, he/she should be positioned with affected side down (at 90-degree angle to bed). Attempt to keep the infant primarily in this position until PIE is resolved.
-
Once PIE or AL has resolved for ≥24 hours, return to optimal lung volume strategy.
-
Patients with PIE and/or gross air leak should be managed with a higher target P aCO 2 range (e.g., 45 to 65 Torr).
HFOV management of patients with hyperinflation and/or CLD
-
Some patients with early CLD have relative hyperinflation. This should be treated by aggressive weaning of Paw and amplitude, as well as by accepting a higher target P aCO 2 range (e.g., 45 to 65 Torr);
-
Hyperinflation may decrease with a lower ventilator frequency;
-
Patients with obvious CLD changes on chest radiograph should also be treated by accepting a higher target P aCO 2 range (e.g., 45 to 65 Torr).
-
If hyperinflation persists, decrease ventilator frequency and simultaneously decrease amplitude to prevent hypocarbia.
Weaning HFOV
The goal is to aggressively wean infants toward extubation;
-
If P aCO 2 remains in target range for >12 hours and patient is stable, wean Δ P by 10%. However, if P aCO 2 is >50 cm H 2O (with normal inflation on CXR) or >60 cm H 20 (with hyperinflation on CXR), weaning is optional;
-
If saturation and lung inflation remain in target range >12 hours, wean Paw by 0.5 to 1.0 cm H 2O, unless this results in an increase in F IO 2 of more than 0.05;
-
Avoid weaning Paw too rapidly, particularly if weaning Paw is associated with increasing F IO 2.
Tidal Ventilation Strategy (SIMV)
Initial SIMV settings
Peak end expiratory pressure (PEEP) 4 to 6 cm H 2O;
-
Tinsp 0.25 to 0.35 second;
-
PIP adequate to give exhaled tidal volume 4 to 7 ml/kg;
-
Rate adjusted to give P aCO 2 in target range, but not to exceed 60 breaths per minute;
-
Flow adequate to deliver PIP.
Adjusting SIMV settings to achieve optimal lung inflation
All of the following changes assume that the exhaled tidal volume is in the target range of 4 to 7 ml/kg. If the dome of the right diaphragm is:
-
Below the 11th rib, decrease PEEP by 2 cm H 2O;
-
Between the 10th and 11th rib, decrease PEEP by 1 cm H 2O;
-
Between 8 and 9.5 ribs, no change in PEEP;
-
Above the eighth rib, increase PEEP by 1 cm H 2O;
-
Above the seventh rib, increase PEEP by 2 cm H 2O;
-
Above the eighth rib, but infant in room air, increasing PEEP is optional;
-
If F IO 2 increases by 0.2 within a 6-hour period, repeat a CXR to evaluate lung inflation.
Adjusting SIMV settings based on P a CO 2
-
P aCO 2 <30 Torr, decrease rate by 20%;
-
P aCO 2 30 to 39 Torr, decrease rate by 10%;
-
P aCO 2 40 to 55 Torr, no change in rate is necessary unless P aCO 2 has been in this range for >12 hours (see SIMV Weaning);
-
P aCO 2 56 to 65 Torr, increase rate by 10%;
-
P aCO 2 >65 Torr, increase rate by 20%;
-
During initial stabilization, repeat arterial blood gas every 20 to 30 minutes until the P aCO 2 is within the target range.
SIMV management of PIE and/or gross air leak with low-lung-volume strategy
-
If the infant has PIE or gross air leak such as pneumothorax or pneumomediastinum, change to a low-lung-volume strategy (diaphragm level between seventh and eighth ribs) accepting whatever F IO 2 is necessary to maintain target blood gases, until the air leak has resolved for ≥24 hours.
-
If the infant has unilateral PIE, he/she should be positioned with affected side down (at 90-degree angle to bed). Attempt to keep the infant primarily in this position until PIE is resolved.
-
Once PIE and/or gross air leak has resolved, return to optimal lung volume strategy. Patients with PIE and/or gross air leak should be managed with a higher target P aCO 2 range (e.g., 45 to 65 Torr).
SIMV management of patient with hyperinflation and/or CLD
-
Some patients with early CLD have relative hyperinflation. This should be treated by aggressive weaning of PEEP and/or PIP, as well as by accepting a higher target P aCO 2 range (e.g., 45 to 65 Torr).
-
If infant is hyperinflated with a tidal volume of 4 ml/kg and high respiratory rate (>40 breaths/min), consider decreasing Tinsp by 0.05 second;
-
Patients with obvious CLD changes on chest radiograph should also be treated by accepting a higher target P aCO 2 range (e.g., 45 to 65 Torr).
Weaning SIMV
The goal is to aggressively wean infants toward extubation.
-
If P aCO 2 remains in target range for >12 hours and patient is stable, wean rate and/or PIP by 10%. However, if P aCO 2 is >50 cm H 2O (with normal inflation on CXR) or >60 cm H 20 (with hyperinflation on CXR), weaning is optional;
-
Weaning may be attempted more frequently than every 12 hours.
Extubation and Reintubation
Extubation will be attempted as soon as the patient is stable on minimal ventilator settings without excess work of breathing.
Extubation will be attempted when patient meets both the following criteria:
-
Paw ≤5 cm H 20 (SIMV) or Paw ≤7 cm H 20 (HFOV);
-
F IO 2 ≤0.25.
Patients who remain stable on these minimal ventilator settings for 6 to 12 hours should be extubated.
Before extubation, infants will be treated with caffeine or theophylline, as prophylaxis for apnea and bradycardia. Infants will be extubated to nasal CPAP (NCPAP) of 4 to 6 cm H 2O (using the Aladdin or Infant-Flow NCPAP system).
-
Initiate NCPAP before extubating the infant;
-
Wean NCPAP by 1 cm H 20 every 24 hours, until infant is on 4 cm H 20 NCPAP;
-
Infant should remain on 4 cm H 20 NCPAP and an F I0 2 of # 0.30 for at least 24 hours, before attempting to discontinue NCPAP and placing infant on nasal cannula or room air.
Patients with the following should be reintubated:
-
Need for more than 8 cm H 2O of nasal CPAP and F IO 2 >0.50 to maintain pulse oximeter saturation in the target range of 88% to 96%;
-
P aCO 2 >65 and pH <7.25;
-
Recurrent apnea and/or bradycardia resulting in oxygen saturation <85%;
Rights and permissions
About this article
Cite this article
Durand, D., Asselin, J., Hudak, M. et al. Early High-Frequency Oscillatory Ventilation Versus Synchronized Intermittent Mandatory Ventilation in Very Low Birth Weight Infants: A Pilot Study of Two Ventilation Protocols. J Perinatol 21, 221–229 (2001). https://doi.org/10.1038/sj.jp.7210527
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.jp.7210527
This article is cited by
-
Peri-extubation settings in preterm neonates: a systematic review and meta-analysis
Journal of Perinatology (2024)
-
Meta-regression analysis of high-frequency ventilation vs conventional ventilation in infant respiratory distress syndrome
Intensive Care Medicine (2007)