Abstract
Purpose
To describe the results of refractive lens exchange (RLE) combined with simultaneous pars plana vitrectomy (PPV) in the management of severe myopia.
Methods
This retrospective study comprised 14 eyes of eight patients who had RLE to treat myopia of −19.0±5.4 diopters (D). Phacoemulsification, posterior chamber intraocular lens (IOL) implantation, and standard three-port vitrectomy were performed. Mean postoperative follow-up time was 30 months (range 12–49).
Results
The postoperative best-corrected visual acuity (BCVA) was 0.68±0.23 compared to 0.37±0.24 preoperatively. There was no postoperative decrease in visual acuity in any eye. Mean postoperative spherical equivalent was −0.7 D (±1.6). At 30 months mean follow-up time, the spherical equivalents of nine eyes (64.3%) were within ±1 D of emmetropia. There was no significant change in astigmatism due to operative procedures. During the 30 months follow-up period three eyes (21.4%) required neodymium : yttrium–aluminium–garnet (Nd : YAG) capsulotomy for posterior capsule opacification. No retinal detachments or cases of cystoid macular oedema (CME) were observed during the follow-up.
Conclusion
RLE was effective in correcting severe myopia. The simultaneously performed PPV may reduce the risk of postoperative retinal detachment. This was a pilot study, to draw definitive conclusions a prospective study has to be initiated.
Similar content being viewed by others
Introduction
Several techniques of refractive surgery are used to reduce high myopia. At present, there are principally three techniques including laser in situ keratomileusis (LASIK), phakic intraocular lens (IOL) implantation, and refractive lens exchange (RLE). These different techniques carry various advantages and disadvantages. The predictability of LASIK falls abruptly when used to correct myopia higher than 12.0 diopters (D). Possible complications are undercorrection, regression, corneal ectasia, corneal flap displacement, intraepithelial cysts, and a deteriorated quality of vision.1, 2 Phakic IOL seem to have two major disadvantages over the long term: endothelial cell loss and cataractogenesis.3 Thus, the interest in removal of the clear crystalline lens is renewed and RLE may be considered for the correction of high myopia.
The advantages of RLE include rapid and predictable visual rehabilitation, stable refraction, no regression of myopia, and often superb optical quality with no irregular astigmatism because the cornea remains intact. Postoperative improvements in visual acuity are stable over a long follow-up period.4 The main disadvantages are loss of accommodation, increased risk of retinal detachment, risk of infection, and cystoid macular oedema (CME).
Although RLE is an effective procedure for high-refractive errors, the risks involved should not be underestimated, and careful preoperative evaluation and patient selection is mandatory.5, 6, 7 All complications of conventional cataract surgery do exist; they are rare but may be severe. A devastating early postoperative complication of cataract surgery is bacterial endophthalmitis (0.08%).8, 9 Late postoperative complications of cataract surgery comprise CME (Irvine–Gass syndrome), opacification of the posterior capsule, and retinal detachment. Extremely rare complications are epithelial ingrowth, a filtering bleb, corneal edema due to vitreo-endothelial contact, and dislocation of the IOL.
Of all objections raised against RLE, the risk of retinal detachment still poses the greatest obstacle to the procedure's acceptance, particularly in myopic patients.4, 10, 11 Risk factors are a defect of the posterior capsule, vitreous loss, axial length, predisposing peripheral retinal degenerations, trauma, and age.12, 13, 14 It has been shown that cataract surgery induces a loss of hyaluronic acid from within the vitreous gel, which in turn causes syneresis and posterior vitreous detachment and hence retinal traction which may result in retinal tear formation. Nearly all pseudophakic detachments are rhegmatogenous, generally occurring at the vitreous base.12, 13, 15 Recent papers provide contradictory results concerning the rate of retinal detachment after RLE compared with the natural rate of retinal detachment in highly myopic eyes (see Table 3).16 In series of Colin et al (1999),17 the incidence of retinal detachment after RLE was nearly double than estimated for persons with myopia greater than −10 D who do not undergo surgery.
Whether retinal prophylaxis (laser photocoagulation of peripheral retinal pathology) might be effective and increase the safety of the procedure or might on the contrary be dangerous has not yet been established. Ripandelli et al18 indicated that potentially blinding complications can occur after RLE, despite prophylactic treatments. Some authors found that more than 25% of the new retinal tears occur in areas previously free of some degeneration.19 Another study has shown that such prophylactic treatment does not reduce, to any significant degree, the incidence of retinal detachment resulting from peripheral degenerations. In many cases, the detachment actually originates at the edges of the prophylactic chorioretinal adhesions.13
Despite advances in surgical technique, retinal detachment remains a major concern after RLE for high myopia. Hovland et al20 showed that if posterior vitreous separation occurs without forming a tear, the risk of retinal detachment decreases significantly. Thus, our study suggests that RLE in combination with pars plana vitrectomy (PPV) can reduce the risk of retinal detachment without increased risk of other complications. In this study, for the first time RLE was routinely combined with simultaneous PPV.
Patients and methods
From February 2000 through October 2002 phacoemulsification and PPV were performed in 14 eyes of eight patients. Patient selection factors included preoperative myopia greater than −13.0 D, contact lens intolerance, occupational needs, and a clear understanding of surgical risks. Axial length and vitreous body status were not taken into account as inclusion criteria. However, the patients often showed obvious vitreous floaters. This facilitates the decision to perform the PPV.21 Table 1 shows the preoperative data of patients who had RLE and simultaneous PPV. There were no patients undergoing RLE without PPV over the same time frame.
No patient had prior retinal detachment. In one patient with lattice degeneration, we performed argon laser photocoagulation 2 months before RLE. We used 200 mW, 400 μm, 0.1 s retinal spots. Three rows of nearly confluent burns were placed immediately around the focal lesion. In another patient intraoperatively a small peripheral retinal tear was detected and laser coagulation was performed during PPV. All eyes showed myopic fundus changes with diffuse or patchy chorioretinal atrophy.
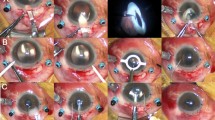
All surgery was performed with general anesthesia. Surgery was bilateral but not simultaneous in six patients. Two patients only got operation of one eye because of severe amblyopia in the fellow eye. There was no benefit expected for the amblyopic eye. Phacoemulsification was performed through a 2.8-mm-wide incision using primary irrigation and aspiration. The incision was widened to 6.0 mm, and a one-piece polymethyl methacrylate (PMMA) IOL with an optical diameter of 6.0 mm (Alcon Laboratories, Inc., Fort Worth, TX, USA) was implanted. This was the standard procedure at that time. In the last three eyes of the study the incision was widened to 3.8 mm only, and a foldable acrylic posterior chamber lens was implanted (Acrysof, Alcon Laboratories, Inc., Fort Worth, TX, USA). In one case with an axial length of 27 mm, we used the SRK-II formula; in all other cases with axial length >28.5 mm, we used the IOLPC-5-formula by Haigis.22 A slight myopia was targeted as a postoperative result. In all cases the IOL was implanted in the capsular bag. The axial length was measured by means of immersion ultrasound biometry.
For the PPV, our surgical approach consisted of a standard three-port vitrectomy. Balanced salt solution was used as an irrigation solution. When necessary, a posterior vitreous detachment was induced by suction with the vitrectomy probe around the optic nerve head. It was not difficult to remove the bulk of the vitreous behind the lens. However, due to vitreous adhesions at the posterior pole, the vitrectomy procedure has to be performed by an experienced surgeon.
The 3-year postoperative evaluation included best-corrected visual acuity, slit-lamp examination, detailed evaluation of the retina through the dilated pupil using a Goldmann three-mirror contact lens, intraocular pressure measurement, and recording of additional procedures including neodymium : yttrium-aluminium-garnet (Nd : YAG) capsulotomy and potential complications.
Results
Refractive and visual results
In all, 14 eyes of eight patients were included in this retrospective study. In some cases, we observed a slight degree of lens opacity preoperatively. Although it did not affect visual acuity (except two eyes), it did induce some refractive changes, further justifying the surgical decision. The mean preoperative spherical equivalent was −19.0 D (±5.4 D). Axial length was between 27 and 36.5 mm, and 28.6% of the implanted IOLs were minus powered (see Table 1). Postoperatively, no significant elevation in intraocular pressure was seen.
At 30 months mean follow-up time, the spherical equivalents of nine eyes (64.3%) were within ±1 D of emmetropia. The mean postoperative spherical equivalent was −0.7 D (±1.6) (see Table 2 and Table 3)
Before operation, best-corrected visual acuity (BCVA) was 0.37±0.24. At 30 months 85.7% of the eyes had a BCVA of 0.5 or better. Mean postoperative BCVA was 0.68±0.23. In all 42.8% of eyes were within 1.0 D of the targeted refractive error, and 85.7%, within 2.0 D. The mean cause for limited visual acuity was the presence of myopic maculopathy. In spite of macular changes 13 eyes (92.9%) got an improvement of the BCVA; one eye had no conversion of BCVA. There was no postoperative decrease in corrected visual acuity in any eye (see Figure 1). The astigmatism was calculated to be 0.93±0.97 D preoperatively and 1.02±0.91 D postoperatively; there was no significant change in astigmatism due to operative procedures (P=0.77).
Nd : YAG capsulotomies
During the 30 months follow-up period three eyes (21.4%) required Nd : YAG capsulotomy for posterior capsule opacification after PMMA lens implantation. There were no complications resulting from capsulotomy. For compensation of possible increase in intraocular pressure patients got a prophylactic dose of oral acetazolamide. An intraoperative capsulotomy was performed in two eyes of one patient because of not removable opacities of the posterior capsule.
Retinal treatments/complications
No intraoperative or postoperative complications (capsular tear, choroidal detachment or bleeding, or CME) occurred. All IOLs were placed in the bag. In one patient with lattice degeneration we performed argon laser photocoagulation 2 months before RLE. In another patient intraoperatively a small peripheral retinal tear was detected and laser coagulation was performed during PPV. There were no cases of retinal detachment in the 30 months follow-up period.
Discussion
As a result of its high predictability, stability, and possibly low morbidity, we believe RLE is a reasonable refractive surgery option for middle-aged patients with high myopia. The main limitation of our retrospective series is size, the numbers are quite small and the conclusions are therefore, fragile. Still, our data and the knowledge from the literature suggest that the major concern after RLE—retinal detachment—can be reduced by simultaneous PPV. This was a pilot study, to draw definitive conclusions a prospective study has to be initiated.
Studies on myopic RLE and cataract extraction reach different conclusions, depending on the patient population and length of follow-up (see Table 4). A survey published by Javitt et al23 in 1994 concluded that patients who have had RLE for myopia could expect a 4.6% chance of moderate visual impairment and a 3.3% chance of severe visual impairment strictly as a result of retinal complications. The study also found evidence that the risk of visual loss is enhanced by bilateral surgery. In a meta-analysis of 13 papers (1996–2003) on myopic RLE and cataract extraction, Richard Packard24 showed that in 1790 eyes, the rate of retinal detachment at 37 months was 1.53%. The results published in 1999 by Colin et al17 showed how the rate of retinal detachment increased with time. In the series of Colin et al, there was no case of retinal deatchment at 1 year, but it was 2% at 4 years, 8% at 7 years and 10% at 10 years. All the patients were treated with phacoemulsification, IOL implantation, and preoperative retinal argon laser prophylaxis when necessary. Colin et al17 also concluded that the need for Nd : YAG capsulotomy after RLE, which was performed in 60% of the cases, increased the risk of retinal complications. Further risk factors included peripheral retinal lesions, the status of the posterior vitreous and a personal or family history of retinal detachments. Lee et al25 followed 24 eyes for 15 months (RLE for myopia of −16.6±1.6 D) and observed no retinal detachments. Similarly, Jimenez-Alfaro et al26 reported no retinal detachments 12–26 months after RLE in 26 eyes with preoperative myopia of −20.8±5.4 D. Lyle et al10 reported no retinal detachments after a mean follow-up of 20 months. Gris et al27 reported one retinal detachment (2.2%) that occurred 4 weeks after surgery in an eye with a posterior capsular tear. Barraquer et al19 found a 7.3% incidence of retinal detachment in a retrospective evaluation of 165 eyes with axial length greater than 26 mm and/or aphakic refractive sphere of 8 D or less. All these result together show that there is the need for a better prophylaxis than argon laser photocoagulation to prevent retinal detachment.
Our study suggests the combination with a PPV to remove the vitreous and to prevent tractions and formation of retinal tears. In our patients no complications due to surgery occurred. We suppose that PPV in the hands of an experienced vitreoretinal surgeon does not increase the risk of operation compared to cataract surgery alone. Peripheral iatrogenic retinal breaks can be a serious complication during vitreous surgery.28 However, only undetected or improperly managed retinal breaks lead to postoperative retinal detachment. The assumption is that the surgeon is experienced in surgery of the anterior and posterior segment of the eye.
Bacterial endophthalmitis is much less common after retinal detachment surgery than following cataract extraction. Thus, this devastating complication seems not to be increased after combined surgery. Also, the risk of suprachoroidal haemorrhage will not be increased through the additional PPV. However, high myopia generally represents a risk faktor for suprachoroidal haemorrhage. No patient showed a cellophane maculopathy or symptoms of a macular pucker. However, for the evaluation of the expansion of macular pucker later follow-up is necessary. In our cases, there were no drawbacks but several considerable advantages due to simultaneous PPV.
It is not clear whether argon laser prophylaxis helps reduce the incidence of retinal detachment after RLE, but it is evident that prophylactic treatment does not always prevent late retinal detachment after RLE. In their review, Ripandelli et al18 reported that retinal breaks occurred along the edge of the photocoagulation in four of the 26 eyes that underwent retinopexy. Colin et al17 reported that a prophylactically treated eye developed retinal detachment from a tear outside the prophylactically treated quadrant. Thus, argon laser prophylaxis is not a confident method to safely prevent retinal detachment. However, PPV eliminates vitreous traction and effectively reduces the risk of detachtment in our study. Hovland et al20 showed that, if posterior vitreous separation occurs without forming retinal tear, the risk of developing retinal detachment is significantly lowered.
Summarizing, we even think that PPV not only reduces the risk of retinal detachment but also reduces the risk of CME. No patient who was operated with RLE and PPV had symptoms of CME. The exact cause of CME following cataract extraction is still unknown, although inflammation, vitreous traction, and generalized vascular incompetence have been postulated as predisposing factors.
Furthermore, we suggest that postoperative YAG-laser capsulotomy had a minimized risk for retinal detachment if the eye had previously got a vitrectomy. The complication of a retinal detachment in patients whose posterior capsule has been accidentally or intentionally disrupted very likely results from the loss of a rigid anterior vitreal barrier. This may be combined with tractional forces applied to areas of vitreo-retinal adhesion, resulting in the formation of peripheral tears.12, 15, 29 Therefore, capsulotomy should be deferred until the patient's impairment caused by capsular opacification warrants the increased risk of retinal complications associated with performance of capsulotomy. Since the risk of retinal detachment is minimized when a vitrectomy is performed simultaneously with RLE, a YAG-capsulotomy can be realized earlier, even if there is only slight visual impairment due to opacification.
Another point of discussion is the application of an intraoperative capsulotomy during PPV.30 Intraoperative capsulotomy may have advantages. Thus, a postoperative laser capsulotomy with potential complications would not be necessary. However, the incidence of postoperative CME can be increased when a primary capsulotomy is performed at the time of lens extraction, because a rupture of the posterior capsule during planned phacoemulisification is a risk factor for CME. However, the risk of CME should not be increased in cases where the vitreous was completely removed during PPV. Otherwise, the postoperative YAG-laser capsulotomy is without a risk of detachment after PPV, so we did not routinely perform an intraoperative capsulotomy in our patients.
In summary, our study suggests that the combination of RLE and PPV has no increased risk of operative and postoperative complications compared to RLE alone. The ‘prophylactic PPV’ may effectively prevent retinal detachment and possibly reduce the appearance of CME. The risk of retinal detachment following YAG-laser capsulotomy can be minimized. However, long and continuous follow-up of the outcomes of RLE combined with PPV for high myopia is absolutely necessary before we can consider this surgical procedure as a routine option. A prospective study including a control group with patients undergoing RLE without PPV should be initiated.
References
Randleman JB, Russell B, Ward MA, Thompson KP, Stulting RD . Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology 2003; 110: 267–275.
Sugar A, Rapuano CJ, Culbertson WW, Huang D, Varley GA, Agapitos PJ et al. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology 2002; 109: 175–187.
O'Brien TP, Awwad ST . Phakic intraocular lenses and refractory lensectomy for myopia. Curr Opin Ophthalmol 2002; 13: 264–270.
Fernandez-Vega L, Alfonso JF, Villacampa T . Clear lens extraction for the correction of high myopia. Ophthalmology 2003; 110: 2349–2354.
Pucci V, Morselli S, Romanelli F, Pignatto S, Scandellari F, Bellucci R . Clear lens phacoemulsification for correction of high myopia. J Cataract Refract Surg 2001; 27: 896–900.
Ceschi GP, Lorenzo FA . Clear lens extraction in high myopic eyes. Klin Monatsbl Augenheilkd 1998; 212: 280–282.
Seward H, Packard R, Allen D . Management of cataract surery in a high myope. Br J Ophthalmol 2001; 85: 1372–1378.
Javitt JC, Street DA, Tielsch JM, Wang Q, Kolb MM, Schien O et al. National outcomes of cataract extration. Ophthalmology 1994; 101: 100–106.
Norregaard JC, Thoning H, Bernth-Petersen P, Andersen TF, Javitt JC, Anderson GF . Risk of endophthalmitis after cataract extration: results from the international cataract surgery outcomes study. Br J Ophthalmol 1997; 81: 102–106.
Lyle WA, Jin GJC . Clear lens extraction for the correction of high refractive error. J Cataract Refract Surg 1994; 20: 273–276.
Goldberg MF . Clear lens extraction for axial myopia. Ophthalmology 1987; 94: 571–582.
Tielsch JM, Legro MW, Cassard SD, Schein OD, Javitt JC, Singer AE et al. Risk factors for retinal detachment after cataract surgery. Ophthalmology 1996; 103: 1537–1545.
Burton TC . The influence of refractive error and lattice degeneration on the incidence of retinal detachment. Trans Am Opththalmol Soc 1990; 87: 143–155.
Boberg-Ans G, Villumsen J, Henning V . Retinal detachment after phakoemulsification cataract extraction. J Cataract Refract Surg 2003; 29: 1333–1338.
Coonan P, Fung WE, Webster RG, Allen Jr AW, Abbott RL . The incidence of retinal detachment following extracapsular cataract extraction. Ophthalmology 1985; 92: 1096–1101.
Ducournau DH, Le Rouic JF . Is pseudophakic retinal detachment a thing of the past in the phacoemulsification era? Ophthalmology 2004; 111: 1069–1070.
Colin J, Robinet A, Cochener B . Retinal detachment after Clear lens extraction for high myopia. Ophthalmology 1999; 106: 2281–2285.
Ripandelli G, Bille B, Fedeli R, Stirpe M . Retinal detachment after Clear lens extraction in 41 eyes with high axial myopia. Retina 1996; 16: 3–6.
Barraquer C, Cavelier C, Mejia LF . Incidence of retinal detachment following Clear-lens extraction in myopic patients. Arch Ophthalmol 1994; 112: 336–339.
Hovland KR . Vitreous findings in fellow eyes of aphakic retinal detachment. Am J Ophthalmol 1978; 86: 350–353.
Hoerauf H, Muller M, Laqua H . Vitreous body floaters and vitrectomy with full visual acuity. Ophthalmologe 2003; 100: 639–643.
Langenbucher A, Haigis W, Seitz B . Difficult lens power calculations. Curr Opin Ophthalmol 2004; 15: 1–9.
Javitt JC . Clear-lens extraction for high myopia—is this an idea whose time has come? Arch Ophthalmol 1994; 112: 321–323.
Packard RB . Refractive lens exchange for myopia—a new perspective? Eye World 2003; 4: 69.
Lee KJ, Lee JH . Long-term results of Clear lens extraction for severe myopia. J Cataract Refract Surg 1996; 22: 1411–1415.
Jimenez-Alfaro I, Miguelez S, Bueno JL, Puy P . Clear lens extraction and implantation of negative-power posterior chamber intraocular lenses to correct extreme myopia. J Cataract Refract Surg 1998; 24: 1310–1316.
Gris O, Güell JL, Manero F, Müller A . Clear lens extraction to correct high myopia. J Cataract Refract Surg 1996; 22: 686–689.
Sjaarda RN, Glaser BM, Thompson JT, Murphy RP, Hanham A . Distribution of iatrogenic retinal breaks in macular hole surgery. Ophthalmology 1995; 102: 1387–1392.
Javitt JC, Tielsch JM, Canner JK, Kolb MM, Sommer A, Steinberg EP . National outcomes of cataract extraction. Ophthalmology 1992; 99: 1487–1498.
Lahey JM, Francis RR, Kearney JJ . Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy. Ophthalmology 2003; 110: 1335–1339.
Acknowledgements
This work was supported by grants from the Sächsisches Ministerium für Wissenschaft und Kunst (SMWK; HWP Programm).
Author information
Authors and Affiliations
Corresponding author
Additional information
None of the authors has a commercial, proprietary, or financial interest in any research or material presented
Rights and permissions
About this article
Cite this article
Uhlmann, S., Wiedemann, P. Refractive lens exchange combined with pars plana vitrectomy to correct high myopia. Eye 20, 655–660 (2006). https://doi.org/10.1038/sj.eye.6701933
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701933
Keywords
This article is cited by
-
IOL-Kalkulation bei hohen Ametropien
Der Ophthalmologe (2008)
-
Komplikationen des refraktiven Linsenaustausches
Der Ophthalmologe (2008)
-
Refractive lens exchange combined with pars plana vitrectomy to correct high myopia
Eye (2006)