Abstract
Background
We have developed a novel application of adapted virtual reality (VR) technology, for the binocular treatment of amblyopia. We describe the use of the system in six children.
Methods
Subjects consisted of three conventional treatment ‘failures’ and three conventional treatment ‘refusers’, with a mean age of 6.25 years (5.42–7.75 years). Treatment consisted of watching video clips and playing interactive games with specifically designed software to allow streamed binocular image presentation.
Results
Initial vision in the amblyopic eye ranged from 6/12 to 6/120 and post-treatment 6/7.5 to 6/24-1. Total treatment time was a mean of 4.4 h. Five out of six children have shown an improvement in their vision (average increase of 10 letters), including those who had previously failed to comply with conventional occlusion.
Conclusions
Improvements in vision were demonstrable within a short period of time, in some children after 1 h of treatment. This system is an exciting and promising application of VR technology as a new treatment for amblyopia.
Similar content being viewed by others
Introduction
Occlusion therapy is a treatment modality that children often find unacceptable, and parents find difficult to implement.1 The prolonged treatment time required for traditional occlusion therapy often means both motivation and compliance can wane leading to a significant failure rate.2, 3 This led to the development of the Interactive Binocular Treatment (I-BiT™) system for amblyopia, a computer-based virtual reality (VR) treatment, which avoids occlusion of the nonamblyopic eye.4 The aim of this case series was to examine the effectiveness of I-BiT™ in improving vision in the amblyopic eyes of six children.
Materials and method
The research I-BiT™ prototype system was set up in a separate clinic room and seven patients (four females and three males, ranging in age from 3 to 7 years) awaiting treatment participated in this tolarability study. All children found the format easy and enjoyable to use. All were able to successfully complete the games and watch the movie clips within the virtual environment. There were no side effects noted during or after I-BiT™ sessions. Both the children and their parents expressed interest and enjoyment in this new method of treatment, and those parents who had previous experience with patching stated they would prefer this treatment modality rather than occlusion therapy. After the demonstration session the child was asked a number of questions about what they thought about using the I-BiT system and the programmes/cartoons they saw and asked what else they would like to do using the system. The parents and children were asked about how long they thought children could use the system during a treatment session and how many times a week they would be prepared to attend. Overall patient and parental attitudes towards the use of the I-BiT system were positive and enthusiastic. Indeed many parents and patients welcomed a treatment option that did not include patching. This was because the child was averse to wearing a patch and parents found occlusion difficult to implement.
Children with residual amblyopia, in whom conventional treatment had failed or was refused, were invited to participate in this active treatment study after there was no further improvement in the vision with the glasses alone. Six consecutive patients, who agreed to attend the hospital for the trial, were enrolled and treated. Coincidentally, they were all males, female children showed an interest in the treatment but those asked were unable to attend the number of sessions required. Amblyopia was defined as an interocular difference of 0.2 log units or greater using the Keeler crowded LogMAR (Glasgow) acuity test. Local ethics committee approval for this study had been granted.
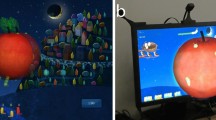
Together with the I-BiT™ system, specific software was designed to allow a VR environment to be viewed binocularly. This allows elements of the visual scene to be preferentially presented to one eye, while maintaining a binocular presentation of the whole virtual scene. A full description of the I-BiT™ system and how it is used is given in a related paper.4 Children attended for one to two treatment sessions a week. Each session consisted of watching a video clip, presented to the amblyopic eye, on a ‘virtual TV’ within the I-BiT™ system, for 20 min. The video clip was presented preferentially to the amblyopic eye with the nonamblyopic eye fixating the TV surround. Specially developed VR games were then used with similar preferential binocular presentation. Each child then played one of the interactive games for several minutes.
Visual acuity was tested, using the Glasgow LogMar crowded acuity cards, before and after each session. As this was purely a pilot study of a new system, vision tests were not randomly selected to eliminate the learning effect. The vision assessments and treatments were conducted by a research orthoptist (PW). The children continued with treatment until visual acuity was stable and no further improvements occurred.
Results
Three patients had not had any previous treatment for their amblyopia, and three patients had failed to improve with conventional occlusion therapy. All patients were male, with a mean age of 6.25 (range 5.42–7.75 years). Patient characteristics and the results following treatment with the I-BiT™ system are shown in Table 1. Pretreatment visual acuity in the amblyopic eye ranged from 6/12 to 6/120, all patients had at least 6/7.5 vision in their better eye. The entry level of vision for this pilot study was not specified, those with gross amblyopia were surprisingly able to watch the TV screen and play the game without suppressing the amblyopic eye. Post-treatment visions ranged from 6/7.5 to 6/30-2. Patients one, two, five, and six had parafoveal fixation, child three had wandering fixation and on child four there was no clear view of the fixation point. There was an overall mean improvement in LogMAR visual acuity of 10 letters (range −3 to 23, see Figure 1). In the five patients who responded to I-BiT™ treatment, there was a mean improvement of 13 letters (3.25 lines on Keeler LogMAR chart, see Figure 1). Visual acuity fluctuated in patient 1 during treatment between 6/9.5 and 6/19, with return of vision to pretreatment levels (6/15) at the 4-week follow-up. Overall, the vision first started to improve within an hour of treatment. No child experienced side effects either during or following treatment with the I-BiT™ system. Statistical analysis was not carried out due to the small number of patients involved in the pilot study. A power analysis was carried out and showed that, due to the changes in pre- and post-treatment visions, eight patients would be adequate for statistical analysis. However, using the method described by Stewart et al,5 which enables the calculation of the proportion of change in vision from pre- to post-treatment, there was an overall mean improvement in vision of 42%.
Discussion
Amblyopia remains an important preventable cause of visual impairment.6, 7 Previous attempts have been made to find alternative therapeutic modalities for amblyopia,8, 9, 10, 11 but none of these have supplanted occlusion. However, noncompliance with occlusion therapy can be problematic and this is well documented.2, 3 Our results have shown evidence of rapid improvements in the vision of five of the six children following only minimal treatment (4.4 h mean total treatment), possibly due to the dynamic nature of the I-BiT™ stimuli. However, the pattern of response appears to be different in each child. Poor presenting visual acuity would appear not to be a barrier to treatment with this system. This is a significant finding as older children, with dense amblyopia, are notoriously challenging to treat with traditional occlusion therapy and are frequently noncompliant. Follow-up in our series has now been up to 22 months. In two children, the vision has remained stable, in two children the vision has improved (one with the use of atropine) and in one the vision has reduced, but not to pretreatment level. Although this preliminary study involves very small numbers, we can demonstrate a positive response to this new treatment modality. The authors are aware that case study results may not generalise to the wider population and these early results must be treated cautiously. Further studies will include a trial of the response of children who have not previously received treatment, consisting of equal numbers of males and females; and assessment of older children in whom occlusion was unsuccessful. These trials will also have a different treatment clinician to the orthoptist assessing the visual acuity. Following on from these studies will be a placebo-controlled trial, as yet the discussion continues on what constitutes a placebo treatment.
This is an exciting new development, which is fun and interactive for children. The VR-based I-BiT™ system, with further development, is a step towards a more ideal, rapid, acceptable, and binocular therapy for amblyopia. Further studies are needed to determine the contribution of our I-BiT™ system in the treatment of amblyopia.
References
Gregson R . Why are we so bad at treating amblyopia? Eye 2002; 16(4): 401.
Newsham D . Parental non-concordance with occlusion therapy. Br J Ophthalmol 2000; 84(9): 957–962.
Stewart CE, Fielder AR, Stephens DA, Moseley MJ . Design of the monitored occlusion treatment of amblyopia study (MOTAS). Br J Ophthalmol 2002; 86: 915–919.
Eastgate RM, Griffiths GD, Waddingham PE, Moody ADR, Butler TKH, Cobb SV et al. Modified virtual reality technology for treatment of amblyopia. Eye 2005, April 15 [E-pub ahead of print].
Stewart CE, Moseley MJ, Fielder AR . Defining and measuring treatment outcome in unilateral amblyopia. Br J Ophthalmol 2003; 87(10): 1229–1231.
Hillis A . Amblyopia: prevalent, curable, neglected. Public Health Rev 1986; 14(3-4): 213–235.
Rahi JS, Dezateux C . Improving the detection of childhood visual problems and eye disorders. Lancet 2002; 359: 1083–1084.
Mohan K, Dhankar V, Sharma A . Visual acuities after Levodopa administration in amblyopia. JPOS 2001; 38: 62–67.
Campbell FW, Hess RF, Watson PG, Banks R . Preliminary results of physiologically based treatment of amblyopia. Br J Ophthalmol 1978; 62: 748–755.
The Pediatric Eye Disease Investigator Group. A randomised trial of atropine vs patching for treatment of moderate amblyopia in children. Arch Ophthalmol 2002; 120(3): 268–278.
Sullivan GD, Fallowfield LJ . A controlled test of the CAM treatment for amblyopia. Br Orthopt J 1980; 37: 47–55.
Acknowledgements
We thank Mr Larry Benjamin, Consultant Ophthalmologist, for his helpful comments on this paper and as an independent assessor for both charity funding and ethics approval. Research funded by William and Mabel Morris Charitable Trust and Sandra Charitable Trust. Additional funding provided by University of Nottingham and Queen's Medical Centre, NHS Trust. Financial interest: Patent application filed jointly with University of Nottingham, Queens Medical Centre NHS Trust and the authors. Poster presented at the Royal College of Ophthalmologists Annual Congress, Birmingham, 2003.Int. Pat. App. WO 03/092482. Technology licensed to Carleton Optical Research Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waddingham, P., Butler, T., Cobb, S. et al. Preliminary results from the use of the novel Interactive Binocular Treatment (I-BiT™) system, in the treatment of strabismic and anisometropic amblyopia. Eye 20, 375–378 (2006). https://doi.org/10.1038/sj.eye.6701883
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701883
Keywords
This article is cited by
-
Rehabilitation of visual functions in adult amblyopic patients with a virtual reality videogame: a case series
Virtual Reality (2023)
-
Study protocol for a randomized controlled trial of the NEIVATECH virtual reality system to improve visual function in children with anisometropic amblyopia
BMC Ophthalmology (2022)
-
Clinical investigation plan for the use of interactive binocular treatment (I-BiT) for the management of anisometropic, strabismic and mixed amblyopia in children aged 3.5–12 years: a randomised controlled trial
Trials (2019)
-
The treatment of amblyopia: current practice and emerging trends
Graefe's Archive for Clinical and Experimental Ophthalmology (2019)
-
A binocular iPad treatment for amblyopic children
Eye (2014)