Abstract
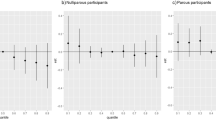
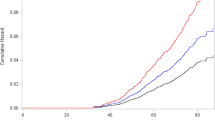
From 1940 through the 1960s, diethylstilbestrol (DES), a synthetic oestrogen, was given to pregnant women to prevent pregnancy complications and losses. Subsequent studies showed increased risks of reproductive tract abnormalities, particularly vaginal adenocarcinoma, in exposed daughters. An increased risk of breast cancer in the DES-exposed mothers was also found in some studies. In this report, we present further follow-up and a combined analysis of two cohorts of women who were exposed to DES during pregnancy. The purpose of our study was to evaluate maternal DES exposure in relation to risk of cancer, particularly tumours with a hormonal aetiology. DES exposure status was determined by a review of medical records of the Mothers Study cohort or clinical trial records of the Dieckmann Study. Poisson regression analyses were used to estimate relative risks (RR) and 95% confidence intervals (CI) for the relationship between DES and cancer occurrence. The study results demonstrated a modest association between DES exposure and breast cancer risk, RR = 1.27 (95% CI = 1.07–1.52). The increased risk was not exacerbated by a family history of breast cancer, or by use of oral contraceptives or hormone replacement therapy. We found no evidence that DES was associated with risk of ovarian, endometrial or other cancer. © 2001 Cancer Research Campaign
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Autunes CMF, Stolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, Burnett L, Rutledge A, Pokempner M and Garcia R (1979) Endometrial cancer and estrogen use. Report of a large case-control study. New Engl J Med 300: 9–13
Beral V and Colwell L (1980) Randomised trial of high doses of stilboestrol and ethisterone in pregnancy: long term follow-up of mothers. B M J 281: 1098–1101
Bibbo M, Haenszel WM, Wied GL, Hubby M and Herbst AL (1978) A twenty-five-year follow-up study of women exposed to diethylstilbestrol during pregnancy. New Engl J Med 298: 763–776
Breslow NE and Day NE (1987) Statistical Methods in Cancer Research II: The Design and Analysis of Cohort Studies. IARC Scientific Publication 94-2789, International Agency for Research on Cancer: Lyon, France
Calle EE, Mervis CA, Thun MJ, Rodriguez C, Wingo PA and Heath CW, Jr (1996) Diethylstilbestrol and risk of fatal breast cancer in a prospective cohort of US women. Am J Epidemiol 144: 645–652
CGHFBC (Collaborative Group on Hormonal Factors in Breast Cancer) (1997) Breast cancer and hormone replacement therapy: collaborative reanalysis of data from 51 epidemiological studies of 52 705 women with breast cancer and 108 411 women without breast cancer. Lancet 350: 1047–1059
Clark LG and Portier KM (1979) Diethylstilbestrol and the risk of cancer. New Engl J Med 300: 263–264
Colditz GA, Hankinson SE, Hunter DJ, Willett WC, Manson JE, Stampfer MJ, Hennekens C, Rosner B and Speizer FE (1995) The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. New Engl J Med 332: 1589–1593
Colton T, Greenberg ER, Noller K, Resseguie L, Van Bennekom C, Heeren T and Zhang Y (1993) Breast cancer in mothers prescribed diethylstilbestrol in pregnancy. J Am Med Assoc 269: 2096–2100
Dieckmann WJ, Davis ME, Rynkiewicz LM and Pottinger RE (1953) Does the administration of diethylstilbestrol during pregnancy have therapeutic value?. Am J Obstet Gynecol 66: 1062–1081
Greenberg ER, Barnes AB, Resseguie L, Barrett JA, Burnside S, Lanza LL, Neff RK, Stevens M, Young RH and Colton T (1984) Breast cancer in mothers given diethylstilbestrol in pregnancy. New Engl J Med 311: 1393–1398
Hadjimichael OC, Meigs JW, Falcier FW, Thompson WD and Flannery JT (1984) Cancer risk among women exposed to exogenous estrogens during pregnancy. J Natl Cancer Inst 73: 831–834
Herbst AL and Scully RE (1971) Adenocarcinoma of the vagina. Association of maternal stilboestrol therapy with tumor appearance in young women. New Engl J Med 284: 878–881
Heston JF, Kelley JAB, Meigs JW and Flannery JT (1986) 45 years of cancer incidence in Connecticut: 1935–1979. NCI Monograph Series no. 70, NIH Publication no. 86-2652, NCI: Bethesda MD
Hoover R, Fraumeni JF, Jr, Everson R and Myers MH (1976) Cancer of the uterine corpus after hormonal treatment for breast cancer. Lancet XX: 885–887
Hoover R, Gray LA and Fraumeni JF, Jr (1977) Stilboestrol (Diethylstilbestrol) and the risk of ovarian cancer. Lancet, 533–534
Hubby MM, Haenszel WM and Herbst AL (1981) Effects on the mother following exposure to diethylstilbestrol during pregnancy. Developmental Effects of Diethylstilbestrol (DES) in Pregnancy, Herbst AL, Bern HA (eds) pp. 120–128, Thieme-Stratton Inc.: New York
Kelsey JL, Thompson WD and Evans AS (1986). Methods in Observational Epidemiology, Oxford University Press: New York
Newbold RR (1993) Gender-related behavior in women exposed prenatally to diethylstilbestrol. Env Health Perspect 101: 208–213
Noller KL and Fish CR (1974) Diethylstylbestrol usage: its interesting past, important present, and questionable future. Med Clin North Am 58: 793–810
Ries LAG, Miller BA, Hankey BF, Kosary CL, Harras A and Edward BK (1999) SEER Cancer Statistics Review, 1973–1996: Tables and Graphs. NIH Publication 99-2789, National Cancer Institute: Bethesda, MD
Schairer C, Lubin J, Troisi R, Sturgeon S, Brinton L and Hoover R (2000) Menopausal estrogen and estrogen-progestin replacement therapy and breast cancer risk. J Am Med Assoc 83: 485–491
Vessey MP, Fairweather DV, Norman-Smith B and Buckley J (1983) A randomized double-blind controlled trial of the value of stilboestrol therapy in pregnancy: long-term follow-up of mothers and their offspring. Br J Obstet Gynecol 90: 1007–1017
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Titus-Ernstoff, L., Hatch, E., Hoover, R. et al. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer 84, 126–133 (2001). https://doi.org/10.1054/bjoc.2000.1521
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1054/bjoc.2000.1521
Keywords
This article is cited by
-
From Wingspread to CLARITY: a personal trajectory
Nature Reviews Endocrinology (2021)
-
Estrogen receptor alpha (ERα)–mediated coregulator binding and gene expression discriminates the toxic ERα agonist diethylstilbestrol (DES) from the endogenous ERα agonist 17β-estradiol (E2)
Cell Biology and Toxicology (2020)
-
State of the evidence 2017: an update on the connection between breast cancer and the environment
Environmental Health (2017)
-
Pregnancy characteristics and maternal breast cancer risk: a review of the epidemiologic literature
Cancer Causes & Control (2010)
-
Dietary fat intake and gestational weight gain in relation to estradiol and progesterone plasma levels during pregnancy: a longitudinal study in Swedish women
BMC Women's Health (2009)