Abstract
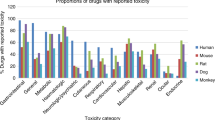
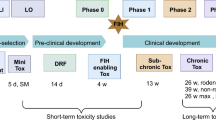
Preclinical toxicology studies are performed prior to phase I trials with novel cancer therapeutics to identify a safe clinical starting dose and potential human toxicities. The primary aim of this study was to evaluate the ability of rodent-only toxicology studies to identify a safe phase I trial starting dose. In addition, the ability of murine studies to predict the quantitative and qualitative human toxicology of cancer therapeutics was studied. Data for 25 cancer drugs were collated for which the preclinical and clinical routes and schedules of administration were either the same (22/25), or closely matched. The maximum tolerated dose/dose lethal to 10% of mice (MTD/LD10) was identified for 24 drugs, and in patients the maximum administered dose (MAD) was associated with dose-limiting toxicity (DLT) in initial clinical trials with 20 compounds. In addition, for 13 agents, the toxicity of the drug at one-tenth the mouse MTD/LD10 was also investigated in rats, following repeated administration (20 doses). A phase I trial starting dose of one-tenth the mouse MTD/LD10 (mg m–2) was, or would have been, safe for all 25 compounds. With the exception of nausea and vomiting, which cannot be assessed in rodents, other common DLTs were accurately predicted by the murine studies (i.e. 7/7 haematological and 3/3 neurological DLTs). For two of the 13 drugs studied in rats, repeated administration of one-tenth the mouse MTD/LD10 was toxic, leading to a reduction in the phase I trial starting dose; however, one-tenth the mouse MTD/LD10 was subsequently tolerated in patients. For the 20 drugs where clinical DLT was reached, the median ratio of the human MAD to the mouse MTD/LD10 was 2.6 (range 0.2–16) and the median ratio of the clinical starting dose to the MAD was 35 (range 2.3–160). In contrast, in 13 subsequent phase I trials with 11 of the initial 25 drugs, the median ratio of the clinical starting dose to the MAD was 2.8 (range 1.6–56), emphasizing the value of early clinical data in rapidly defining the dose range for therapeutic studies. For all 25 drugs studied, rodent-only toxicology provided a safe and rapid means of identifying the phase I trial starting dose and predicting commonly encountered DLTs. This study has shown that the routine use of a non-rodent species in preclinical toxicology studies prior to initial clinical trials with cancer therapeutics is not necessary.
Similar content being viewed by others
Article PDF
Change history
16 November 2011
This paper was modified 12 months after initial publication to switch to Creative Commons licence terms, as noted at publication
References
Arbuck, SG (1996) Workshop on Phase I study design. Ann Oncol 7: 567–573.
Betteridge, RF, Bosanquet, AG & Gilby, ED (1990) Pharmacokinetics of 2,5-diaziridinyl-3,6-bis(2-hydroxyethylamino)-1,4-benzoquinone (BZQ, NSC224070) during a Phase I clinical trial. Eur J Cancer 26: 107–112.
Burtles, SS, Newell, DR, Henrar, REC & Connors, TA (1995) Revisions of general guidelines for the preclinical toxicology of new cytotoxic anticancer agents in Europe. Eur J Cancer 31A: 408–410.
Carmichael, J, Cantwell, BMJ, Mannix, KA, Veale, D, Elford, HL, Blackie, R, Kerr, DJ, Kaye, SB & Harris, AL (1990) A Phase I and pharmacokinetic study of didox administered by 36 hour infusion. Br J Cancer 61: 447–450.
Collins, JM, Zaharko, DS, Dedrick, RL & Chabner, BA (1986) Potential roles for preclinical pharmacology in Phase I clinical trials. Cancer Treat Rep 70: 73–80.
Collins, JM, Grieshaber, CK & Chabner, BA (1990) Pharmacologically guided Phase I clinical trials based upon preclinical drug development. J Natl Cancer Inst 82: 1321–1326.
Decoster, G, Stein, G & Holdener, EE (1990) Responses and toxic deaths in Phase I clinical trials. Ann Oncol 1: 175–181.
DeGeorge, JJ, Ahn, C-H, Andrews, PA, Brower, ME, Giorgio, DW, Goheer, ME, Lee-Ham, DY, McGuinn, WD, Schmidt, W, Sun, CJ & Tripathi, SC (1998) Regulatory considerations for preclinical development of anticancer drugs. Cancer Chemother Pharmacol 41: 173–185.
Dent, SF & Eisenhauer, EA (1996) Phase I trial design: are new methodologies being put into practise?. Ann Oncol 7: 561–566.
EORTC Pharmacokinetics and Metabolism Group (1987) Pharmacokinetically guided dose escalation in Phase I clinical trials. Eur J Cancer 23: 1083–1087.
Estey, E, Hoth, D, Simon, R, Marsoni, S, Leyland-Jones, B & Wittes, R (1986) Therapeutic response in Phase I trials of antineoplastic agents. Cancer Treat Rep 70: 1105–1115.
Foster, BJ, Newell, DR, Graham, MA, Gumbrell, LA, Jenns, KE, Kaye, SB & Calvert, AH (1992) Phase I trial of the anthrapyrazole CI-941: prospective evaluation of pharmacologically guided dose-escalation. Eur J Cancer 28: 463–469.
Foster, BJ, Newell, DR, Carmichael, J, Harris, AL, Gumbrell, LA, Jones, M, Gogard, PM & Calvert, AH (1993a) Preclinical, Phase I and pharmacokinetic studies with the dimethyl phenyltriazene CB10-277. Br J Cancer 67: 362–368.
Foster, BJ, Newell, DR, Gumbrell, LA, Jenns, KE & Calvert, AH (1993b) Phase I trial with pharmacokinetics of CB10-277 given by 24 hours continuous infusion. Br J Cancer 67: 369–373.
Freireich, EJ, Gehan, EA, Rall, DP, Schmidt, LH & Skipper, HE (1966) Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Rep 50: 219–244.
Goldsmith, MA, Slavik, M & Carter, SK (1975) Quantitative prediction of drug toxicity in humans from toxicology in small and large animals. Cancer Res 35: 1354–1364.
Grieshaber, CK & Marsoni, S (1986) Relationship of preclinical toxicology to findings in early clinical trials. Cancer Treat Rep 70: 65–72.
Homan, ER (1972) Quantitative relationships between toxic doses of antitumour chemotherapeutic agents in animals and man. Cancer Chemother Rep Part 3 3: 13–19.
Horwich, A, Holliday, SB, Deacon, JM & Peckham, MJ (1986) A toxicity and pharmacokinetic study in man of the hypoxic-cell radiosensitizer RSU-1069. Br J Radiol 59: 1238–1240.
Jayson, GC, Crowther, D, Prendiville, J, McGown, AT, Scheid, C, Stern, P, Young, R, Brenchley, P, Chang, J, Owens, S & Pettit, GR (1995) A Phase I trial of bryostatin 1 in patients with advanced malignancy using a 24 hour intravenous infusion. Br J Cancer 72: 461–468.
Jodrell, DI, Bowman, A, Stewart, M, Dunlop, N, French, R, MacLellan, A, Cummings, J & Smyth, JF (1998) Dose-limiting neurotoxicity in a Phase I study of penclomedine (NSC 388720, CRC 88-04), a synthetic α-picoline derivative, administered intravenously. Br J Cancer 77: 808–811.
Joint Steering Committee of the EORTC and CRC (1990). General guidelines for the preclinical toxicology of new cytotoxic anticancer agents in Europe. Eur J Cancer 26: 411–414
Judson, IR, Calvert, AH, Rutty, CJ, Abel, G, Gumbrell, LA, Graham, MA, Evans, BD, Wilman, DEV, Ashley, SE & Cairnduff, F (1989) Phase I trial and pharmacokinetics of trimelamol (N2 N4 N6-trihydroxymethyl-N2 N4 N6-trimethylmelamine). Cancer Res 49: 5475–5479.
Judson, I, Briasoulis, E, Raynaud, F, Hanwell, J, Berry, C & Lacey, H (1997) Phase I trial and pharmacokinetics of the tubulin inhibitor 1069C85, a synthetic agent binding at the colchicine site designed to overcome multidrug resistance. Br J Cancer 75: 608–613.
Kerr, DJ, Kaye, SB, Graham, J, Cassidy, J, Harding, M, Setanoians, A, McGrath, JC, Vezin, WR, Cunningham, D, Forrest, G & Soukop, M (1986) Phase I and pharmacokinetic study of LM975 (flavone acetic acid ester). Cancer Res 46: 3142–3146.
Kerr, DJ, Kaye, SB, Cassidy, J, Bradley, C, Rankin, EM, Adams, L, Setanoians, A, Young, T, Forrest, G, Soukop, M & Clavel, M (1987) Phase I and pharmacokinetic study of flavone acetic acid. Cancer Res 47: 6776–6781.
McKeage, MJ, Mistry, P, Ward, J, Boxall, FE, Loh, S, O’Neill, C, Ellis, P, Kelland, LR, Morgan, SE, Murrer, B, Santabarbara, P, Harrap, KR & Judson, IR (1995) A Phase I and pharmacology study of an oral platinum complex, JM216: dose-dependent pharmacokinetics with single-dose administration. Cancer Chemother Pharmacol 36: 451–458.
McKeage, MJ, Raynaud, F, Ward, J, Berry, C, O’Dell, D, Kelland, LR, Murrer, B, Santabarbara, P, Harrap, KR & Judson, IR (1997) Phase I and pharmacokinetic study of an oral platinum complex given daily for 5 days in patients with cancer. J Clin Oncol 15: 2691–2700.
Mick, R & Ratain, MJ (1993) Model-guided determination of maximum tolerated dose in Phase I clinical trials: evidence for increased precision. J Natl Cancer Inst 85: 217–223.
Millward, MJ, Newell, DR, Mummaneni, V, Igwemezie, LN, Balmanno, K, Charlton, CJ, Gumbrell, L, Lind, MJ, Chapman, F, Proctor, M, Simmonds, D, Cantwell, BMJ, Taylor, GA, McDaniel, C, Winograd, B, Kaul, S, Barbaiya, RH & Calvert, AH (1995) Phase I and pharmacokinetic study of the water-soluble etoposide prodrug, etoposide phosphate (BMY-40481). Eur J Cancer 31A: 2409–2411.
Newlands, ES, Blackledge, G, Slack, JA, Goddard, C, Brindley, CJ, Holden, L & Stevens, MFG (1985) Phase I clinical trial of mitozolomide. Cancer Treat Rep 69: 801–805.
Newlands, ES, Blackledge, GRP, Slack, JA, Rustin, GJS, Smith, DB, Stuart, NSA, Quarterman, CP, Hoffman, R, Stevens, MFG, Brampton, MH & Gibson, AC (1992) Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856). Br J Cancer 65: 287–291.
Newlands, ES, Rustin, GJS & Brampton, MH (1996) Phase I trial of elactocin. Br J Cancer 74: 648–649.
O’Quigley, J & Chevret, S (1991) Methods for dose finding studies in cancer clinical trials: a review and results of a Monte Carlo study. Stat Med 10: 1647–1664.
Penta, JS, Rozencweig, M, Guarino, AM & Muggia, FM (1979) Mouse and large-animal toxicology studies of twelve antitumour agents: relevance to starting dose for Phase I clinical trials. Cancer Chemother Pharmacol 3: 97–101.
Penta, JS, Rosner, GL & Trump, DL (1992) Choice of starting dose and escalation for Phase I studies of antitumour agents. Cancer Chemother Pharmacol 31: 247–250.
Phillip, PA, Rea, D, Thavasu, P, Carmichael, J, Stuart, NSA, Rockett, H, Talbot, DC, Ganesan, T, Pettit, GR, Balkwill, F & Harris, AL (1993) Phase I study of bryostatin 1: assessment of interleukin 6 and tumour necrosis factor α induction in vivo. J Natl Cancer Inst 85: 1812–1818.
Prendiville, J, Crowther, D, Thatcher, N, Woll, PJ, Fox, BW, McGown, A, Testa, N, Stern, P, McDermott, R, Potter, M & Pettit, GR (1993) A Phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br J Cancer 68: 418–424.
Rafi, I, Taylor, GA, Calvete, JA, Boddy, AV, Balmanno, K, Bailey, N, Lind, M, Calvert, AH, Webber, S, Jackson, RJ, Johnston, A, Clendeninn, N & Newell, DR (1995) Clinical pharmacokinetic and pharmacodynamic studies with the nonclassical antifolate thymidylate synthase inhibitor 3,4-dihydro-2-amino-6-methyl-4-oxo-5-(4-pyridylthio)-quinazoline dihydrochloride (AG337) given by 24-hour intravenous infusion. Clin Cancer Res 1: 1275–1284.
Rafi, I, Boddy, AV, Calvete, JA, Taylor, GA, Newell, DR, Bailey, NP, Lind, MJ, Green, M, Hine, J, Johnston, A, Clendeninn, N & Calvert, AH (1998) Preclinical and Phase I clinical studies with the non-classical antifolate thymidylate synthase inhibitor nolatrexed dihydrochloride given by prolonged administration in patients with solid tumours. J Clin Oncol 16: 1131–1141.
Ratain, MJ, Mick, R, Schilsky, RL & Siegler, M (1993) Statistical and ethical issues in the design and conduct of Phase I and Phase II clinical trials of new anticancer agents. J Natl Cancer Inst 85: 1637–1643.
Rozencweig, M, Von Hoff, DD, Staquet, MJ, Schein, PS, Penta, JS, Goldin, A, Muggia, FM, Freireich, EJ & DeVite, VT (1981) Animal toxicology for early clinical trials with anticancer agents. Cancer Clin Trials 4: 21–28.
Simon, R, Freidlin, B, Rubinstein, L, Arbuck, S, Collins, J & Christian, MC (1997) Accelerated titration designs for Phase I clinical trials in oncology. J Natl Cancer Inst 89: 1138–1147.
Smith, DB, Fox, BW, Thatcher, N, Steward, WP, Scarffe, JH, Wagstaff, J, Vezin, R & Crowther, D (1987) Phase I clinical trial of methylene dimethyl sulfonate. Cancer Treat Rep 71: 817–820.
Smith, DB, Ewen, C, Mackintosh, J, Fox, BW, Thatcher, N, Scarffe, JH, Vezin, R & Crowther, D (1988) A Phase I trial and pharmacokinetic study of amphethinile. Br J Cancer 57: 623–627.
Stuart, NSA, Crawford, SM, Blackledge, GRP, Newlands, ES, Slack, J, Hoffman, R & Stevens, MFG (1989) A Phase I study of meta-azidopyrimethamine ethanesulphonate (MZPES): a new dihydrofolate reductase inhibitor. Cancer Chemother Pharmacol 23: 308–310.
Vasey, PA, Kaye, SB, Morrison, R, Twelves, C, Wilson, P, Duncan, R, Thomson, AH, Murray, LS, Hilditch, TE, Murray, T, Burtles, S, Fraier, D, Frigerio, E & Cassidy, J (1999). Clin Cancer Res 5: 83–94.
Veale, D, Carmichael, J, Cantwell, BMJ, Elford, HL, Blackie, R, Kerr, DJ, Kaye, SB & Harris, AL (1988) A Phase I and pharmacokinetic study of didox: a ribonucleotide reductase inhibitor. Br J Cancer 58: 70–72.
Verweij, J (1996) Starting dose levels for Phase I studies. Ann Oncol 7, (suppl.1) 13
Author information
Authors and Affiliations
Rights and permissions
From twelve months after its original publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Newell, D., Burtles, S., Fox, B. et al. Evaluation of rodent-only toxicology for early clinical trials with novel cancer therapeutics. Br J Cancer 81, 760–768 (1999). https://doi.org/10.1038/sj.bjc.6690761
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bjc.6690761
Keywords
This article is cited by
-
A triple combination gemcitabine + romidepsin + cisplatin to effectively control triple-negative breast cancer tumor development, recurrence, and metastasis
Cancer Chemotherapy and Pharmacology (2021)
-
Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials
British Journal of Cancer (2020)
-
A systematic investigation of the maximum tolerated dose of cytotoxic chemotherapy with and without supportive care in mice
BMC Cancer (2017)
-
How do researchers decide early clinical trials?
Medicine, Health Care and Philosophy (2016)
-
Housing temperature-induced stress drives therapeutic resistance in murine tumour models through β2-adrenergic receptor activation
Nature Communications (2015)