Abstract
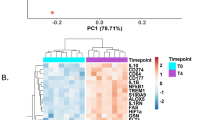
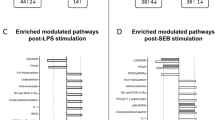
Two shock-inducing toxins that result in similar eventual outcome of disease were studied to determine host gene expression responses, for correlation of both similar and unique gene patterns. We initially used differential display (DD)-PCR and identified 859 cDNA fragments that were differentially expressed after 16 h of in vitro exposure of human peripheral blood mononuclear cells (PBMC) to staphylococcal enterotoxin B (SEB). Upon further examination using custom cDNA microarrays and RT-PCR analysis, we found unique set of genes to each toxin (SEB or lipopolysaccharide (LPS)), especially at early time periods. By 16 h, there was a convergence of some gene expression responses and many of those genes code for proteins such as proteinases, transcription factors, vascular tone regulators, and respiratory distress. In an attempt to replicate the findings in vivo, monkeys were challenged with SEB and the resultant gene expression responses indicated a pattern typical of SEB exposure when compared to LPS, with a similar outcome. We provide evidence that vastly diverse global gene analysis techniques used in unison can not only effectively identify pathogen-specific genomic markers and provide a solid foundation to mechanistic insights but also explain some of the toxin-related symptoms through gene functions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 digital issues and online access to articles
$119.00 per year
only $19.83 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Dinges M, Orwin M, Schlievert P . Exotoxins of Staphylococcus aureus. Clin Microbiol Rev 2000; 13: 16–64.
Jett M, Ionin B, Das R, Neill R . The staphylococcal enterotoxins. In: Sussman M (ed). Molecular Medical Microbiology. Academic Press: San Diego, CA, 2001, pp 1089–1116.
Normann SJ, Jaeger RF, Johnsey RT . Pathology of experimental enterotoxemia. The in vivo localization of staphylococcal enterotoxin B. Lab Invest 1969; 20: 17–25.
Mattix ME, Hunt RE, Wilhelmsen CL, Johnson AJ, Baze WB . Aerosolized staphylococcal enterotoxin B-induced pulmonary lesions in rhesus monkeys (Macaca mulatta). Toxicol Pathol 1995; 23: 262–268.
Hunt RE, Johnson AJ, Jett M et al. Host respiratory protection for lethal staphylococcal enterotoxin B71. In: Kamely D, Bannister KA, Sasmore RM (eds). Army Science: The New Frontiers. Military and Civilian Applications. Borg Biomedical Books: Saratoga, WY, 1993, pp 71–78.
Karima R, Matsumoto S, Higashi H, Matsushima K . The molecular pathogenesis of endotoxic shock and organ failure. Mol Med Today 1999; 5: 123–132.
Schwetz B . From the food and drug administration. JAMA 2002; 287: 33.
Bernard GR, Ely EW, Wright TJ et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med 2001; 344: 699–709.
Heine H, Rietschel ET, Ulmer AJ . The biology of endotoxin. Mol Biotechnol 2001; 19: 279–296.
Ulmer AJ, Flad H, Rietschel T et al. Induction of proliferation and cytokine production in human T lymphocytes by lipopolysaccharide (LPS). Toxicology 2000; 152: 37–45.
Beno DW, Uhing MR, Goto M et al. Staphylococcal enterotoxin B potentiates LPS-induced hepatic dysfunction in chronically catheterized rats. Am J Physiol Gastrointest Liver Physiol 2001; 280: 866–872.
McKay DM, Lu J, Jedrzkiewicz S et al. Nitric oxide participates in the recovery of normal jejunal epithelial ion transport following exposure to the superantigen, Staphylococcus aureus enterotoxin B. J Immunol 1999; 163: 4519–4526.
Opal SM, Cross AS . Clinical trials for severe sepsis. Past failures, and future hopes. Infect Dis Clin North Am 1999; 13: 285–297.
Boldrick J, Alizadeh AA, Diehn M et al. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci USA 2002; 99: 972–977.
Cattaruzza M, Schafer K, Hecker M . Cytokine-induced down-regulation of zfm1/splicing factor-1 promotes smooth muscle cell proliferation. J Biol Chem 2002; 277: 6582–6589.
Newton R, Kuitert LM, Slater DM et al. Cytokine induction of cytosolic phospholipase A2 and cyclooxygenase-2 mRNA is suppressed by glucocorticoids in human epithelial cells. Life Sci 1997; 60: 67–78.
Mendis C, Das R, Jett M . Gene Expression studies in lymphoid cells using differential display. In: Appasani K (ed). Perspectives in Gene Expression. Eaton Publishing: Westborough, MA, 2003.
Wong K, Cheng R, Mok S . Identification of differentially expressed genes from ovarian cancer cells by MICROMAX cDNA microarray system. BioTechniques 2001; 30: 670–675.
Krakauer T, Pitt L, Hunt RE . Detection of interleukin-6 and interleukin-2 in serum of rhesus monkeys exposed to a nonlethal dose of staphylococcal enterotoxin B. Military Med 1997; 162: 612–615.
Berridge MJ . Lymphocyte activation in health and disease. Crit Rev Immunol 1997; 17: 155–178.
Nakashima K, Taga T . Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol Neurobiol 2002; 25: 233–244.
Nakagawa TY, Rudensky AY . The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunol Rev 1999; 172: 121–129.
Brandt E, Peterson F, Ludwig A et al. The beta-thromboglobulins and platelet factor 4: blood platelet-derived CXC chemokines with divergent roles in early neutrophil regulation. J Leukoc Biol 2000; 67: 471–478.
Wang G, Jiang B, Rue E, Semenza G . Hypoxia-inducible factor 1 is a basic-helix–loop–helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92: 5510–5514.
Semenza GL . HIF-1, O(2), and the 3 PHDs: how animal cells signal hypoxia to the nucleus. Cell 2001; 107: 1–3.
Bourne H . Do GTPases direct membrane traffic in secretion? Cell 1988; 53: 669–671.
Li H, Llera A, Mariuzza RA . Structure–function studies of T-cell receptor-superantigen interactions. Immunol Rev 1998; 163: 177–186.
Anderson P . Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev 1997; 61: 33–46.
Eisen M, Spellman PT, Brown PO, Botstein D . Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 1998; 95: 14863–14868.
Acknowledgements
This work was supported by a Grant #DARPA-021-99, from Defense Advance Research Project Agency (DARPA). We like to extend our gratitude to Michael Hartl and Carla Sanchez (Walter Reed Army Institute of Research, Silver Spring, MD 20910, USA) for their technical expertise and diligent work in carrying out the DD-PCR procedure.
Research was conducted in compliance with the Animal Welfare Act and other federal statutes and regulations relating to animals and experiments involving animals and adheres to principles stated in the Guide for the Care and Use of Laboratory Animals, NRC Publication, 1996 edition.
Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the US Army.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mendis, C., Das, R., Hammamieh, R. et al. Transcriptional response signature of human lymphoid cells to staphylococcal enterotoxin B. Genes Immun 6, 84–94 (2005). https://doi.org/10.1038/sj.gene.6364160
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gene.6364160
Keywords
This article is cited by
-
Transcriptome characterization of immune suppression from battlefield-like stress
Genes & Immunity (2013)