Abstract
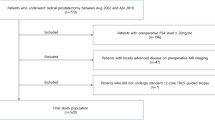
The aim of the study was to prospectively assess the role of apical soft tissue biopsies in radical perineal prostatectomy (RPP) patients with documented apical prostate cancer (PCA) involvement. Between June 1998 and May 1999, 77 consecutive men with localized PCA and documented invasion of the prostatic apex underwent RPP by a single surgeon. Soft tissue biopsies were systematically obtained from the prostatic fossa overlying the apex at the time of surgery. Time to biochemical failure was calculated using the Kaplan–Meier method. The rates of positive apical margins and positive apical soft tissue biopsies were 23.4% (18/77) and 15.6% (12/77). The sensitivity, specificity and positive predictive value of positive apical margins for residual apical disease as determined by apical soft tissue biopsy were 41.7, 80, and 28%, respectively. The overall biochemical failure rate was 28.6% (22/77) with a median follow-up of 51 months (range 3–73 months). The 36-month biochemical recurrence-free survival rate was 55.9±14.9% for patients with positive apical biopsies and 78.7±5.3% for those with negative biopsies (P=0.023). In conclusion, positive apical soft tissue biopsy is an independent predictor of biochemical failure in patients with apical PCA who undergo RPP. Positive apical surgical margins poorly predict residual apical disease that is frequently identifiable by apical soft tissue biopsy. Apical soft tissue biopsies should therefore be obtained in patients with known extensive apical cancer involvement at the time of RPP.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Weldon VE, Tavel FR, Neuwirth H, Cohen R . Patterns of positive specimen margins and detectable prostate specific antigen after radical perineal prostatectomy. J Urol 1995; 153: 1565–1569.
Stamey TA, Villers AA, McNeal JE, Link PC, Freiha FS . Positive surgical margins at radical prostatectomy: importance of the apical dissection. J Urol 1990; 143: 1166–1172; discussion 1172–1173.
Wieder JA, Soloway MS . Incidence, etiology, location, prevention and treatment of positive surgical margins after radical prostatectomy for prostate cancer. J Urol 1998; 160: 299–315.
Obek C, Sadek S, Lai S, Civantos F, Rubinowicz D, Soloway MS . Positive surgical margins with radical retropubic prostatectomy: anatomic site-specific pathologic analysis and impact on prognosis. Urology 1999; 54: 682–688.
Fesseha T, Sakr W, Grignon D, Banerjee M, Wood Jr DP, Pontes JE . Prognostic implications of a positive apical margin in radical prostatectomy specimens. J Urol 1997; 158: 2176–2179.
Rabbani F, Bastar A, Fair WR . Site specific predictors of positive margins at radical prostatectomy: an argument for risk based modification of technique. J Urol 1998; 160: 1727–1733.
Soulie M, Seguin P, Benoit J, Escourrou G, Tollon C, Vazzoler N et al. Impact of a modified apical dissection during radical retropubic prostatectomy on the occurrence of positive surgical margins: a comparative study in 212 patients. Urology 2001; 58: 217–221.
Holzbeierlein JM, Porter II HJ, Thrasher JB . The craft of urologic surgery: modern radical perineal prostatectomy. Urol Clin North Am 2004; 31: 629–641, xi–xii.
Janoff DM, Parra RO . Contemporary appraisal of radical perineal prostatectomy. J Urol 2005; 173: 1863–1870.
Salomon L, Anastasiadis AG, Levrel O, Katz R, Saint F, de la Taille A et al. Location of positive surgical margins after retropubic, perineal, and laparoscopic radical prostatectomy for organ-confined prostate cancer. Urology 2003; 61: 386–390.
Boccon-Gibod L, Ravery V, Vordos D, Toublanc M, Delmas V, Boccon-Gibod L . Radical prostatectomy for prostate cancer: the perineal approach increases the risk of surgically induced positive margins and capsular incisions. J Urol 1998; 160: 1383–1385.
Paulson DF . Radical perineal prostatectomy. Urol Clin North Am 1980; 7: 847–853.
Bostwick DG, Foster CS . Examination of radical prostatectomy specimens:therapeutic and prognostic significance. In: Bostwick FA (ed). Pathology of the Prostate. W.B. Saunders: Philadelphia, 1998, pp 172–189.
Sobin LH, Fleming ID . TNM classification of malignant tumors, fifth edition (1997). Union Internationale Contre le Cancer and the American Joint Committee on Cancer. Cancer 1997; 80: 1803–1804.
Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H . Defining prostate specific antigen progression after radical prostatectomy: what is the most appropriate cut point? J Urol 2001; 165: 1146–1151.
Kaplan EL, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53: 457–481.
Cox DR . Regression models and life tables. J Roy Stat Soc 1972; 34: 187–220.
Shah O, Melamed J, Lepor H . Analysis of apical soft tissue margins during radical retropubic prostatectomy. J Urol 2001; 165 (6 Part 1): 1943–1948; discussion 1948–1949.
Ohori M, Abbas F, Wheeler TM, Kattan MW, Scardino PT, Lerner SP . Pathological features and prognostic significance of prostate cancer in the apical section determined by whole mount histology. J Urol 1999; 161: 500–504.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kübler, H., Szukala, S., Madden, J. et al. Apical soft tissue biopsies predict biochemical failure in radical perineal prostatectomy patients with apical cancer involvement. Prostate Cancer Prostatic Dis 10, 72–76 (2007). https://doi.org/10.1038/sj.pcan.4500926
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500926