Abstract
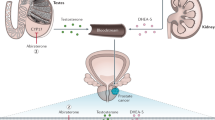
Combination hormonal therapy, comprising a luteinising hormone-releasing hormone analogue (LHRHa) with an antiandrogen, is widely used in the treatment of advanced prostate cancer. There is ongoing debate regarding the use of combination hormonal therapy as opposed to LHRHa monotherapy. The pivotal consideration is whether there are adequate benefits with combination hormonal therapy in terms of increased survival and decreased disease progression to outweigh the increased risk of adverse events and additional cost. The most recent meta-analysis by the Prostate Cancer Trialists' Collaborative Group indicates a small but statistically significant survival benefit with combination hormonal therapy using nonsteroidal antiandrogens. It is, however, noteworthy that combined conclusions derived from such meta-analyses may not apply across each of the individual antiandrogens. Individual studies have reported differences between antiandrogens in terms of both tolerability and efficacy—for example, bicalutamide has been shown to be better tolerated than flutamide, and may be associated with improved survival. In addition, it is essential that treatment decisions are taken in consultation with the patient. Owing to an increasing proportion of cases presenting with early-stage disease, combination hormonal therapy is increasingly used in the neoadjuvant or adjuvant setting with radiotherapy and, in cases of prostate-specific antigen recurrence after prior localised therapy. Further data are awaited to optimise the use of combination hormonal therapy in these new settings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Huggins C, Hodges CV . Studies on prostate cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatase in metastatic carcinoma of the prostate. Cancer Res 1941; 1: 293–297.
Denis LJ . Maximal androgen blockade: facts and fallacies. Endocr Relat Cancer 1998; 5: 353–356.
Anonymous. Leuprolide versus diethylstilbestrol for metastatic prostate cancer. The Leuprolide Study Group. N Engl J Med 1984; 311: 1281–1286.
Kaisary AV, Tyrrell CJ, Peeling WB, Griffiths K . Comparison of LHRH analogue (Zoladex) with orchiectomy in patients with metastatic prostatic carcinoma. Br J Urol 1991; 67: 502–508.
Schmitt B et al. Combined androgen blockade with nonsteroidal antiandrogens for advanced prostate cancer: a systematic review. Urology 2001; 57: 727–732.
Crawford ED et al. A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 1989; 321: 419–424.
Denis LJ et al. Goserelin acetate and flutamide versus bilateral orchiectomy: a phase III EORTC trial (30853). EORTC GU Group and EORTC Data Center. Urology 1993; 42: 119–129, discussion 129–130.
Anonymous. Maximum androgen blockade in advanced prostate cancer: an overview of 22 randomised trials with 3283 deaths in 5710 patients. Prostate Cancer Trialists' Collaborative Group. Lancet 1995; 346: 265–269.
Bennett CL et al. Maximum androgen-blockade with medical or surgical castration in advanced prostate cancer: a meta-analysis of nine published randomized controlled trials and 4128 patients using flutamide. Prostate Cancer P Dis 1999; 2: 4–8.
Anonymous. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet 2000; 355: 1491–1498.
Samson DJ et al. Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 2002; 95: 361–376.
Schellhammer PF et al. Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology 1997; 50: 330–336.
Fisher LD, Gent M, Buller HR . Active-control trials: how would a new agent compare with placebo? A method illustrated with clopidogrel, aspirin, and placebo. Am Heart J 2001; 141: 26–32.
Rothmann M et al. Design and analysis of non-inferiority mortality trials in oncology. Stat Med 2003; 22: 239–264.
Klotz L, Schellhammer P, Carroll K . A re-assessment of the role of combined androgen blockade for advanced prostate cancer. BJU Int 2004; 93: 1177–1182.
Soloway MS . Combined androgen blockade: an optimal therapy for minimally advanced prostate cancer? Br J Urol 1998; 81: 87–94, discussion 94–95.
Soloway MS et al. Bicalutamide and flutamide, each in combination with luteinizing hormone-releasing hormone analogs, in advanced prostate cancer: exploratory analysis of impact of extent of disease by bone scan on outcome. Prostate J 2000; 2: 137–145.
Kelly WK . Endocrine withdrawal syndrome and its relevance to the management of hormone refractory prostate cancer. Eur Urol 1998; 34 (Suppl 3): 18–23.
Klotz L . Combined androgen blockade in prostate cancer: meta-analyses and associated issues. BJU Int 2001; 87: 806–813.
Schellhammer PF et al. Prostate specific antigen decreases after withdrawal of antiandrogen therapy with bicalutamide or flutamide in patients receiving combined androgen blockade. J Urol 1997; 157: 1731–1735.
Joyce R et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol 1998; 159: 149–153.
Scher HI et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. J Clin Oncol 1997; 15: 2928–2938.
Schmitt B et al. Maximal androgen blockade for advanced prostate cancer (Cochrane Review). In: The Cochrane Library, Issue 4. John Wiley & Sons, Ltd: Chichester, UK, 2003.
Aprikian AG, Fleshner N, Langleben A, Hames J . An oncology perspective on the benefits and cost of combined androgen blockade in advanced prostate cancer. Can J Urol 2003; 10: 1986–1994.
Schellhammer PF . An evaluation of bicalutamide in the treatment of prostate cancer. Expert Opin Pharmacother 2002; 3: 1313–1328.
Eisenberger MA et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998; 339: 1036–1042.
Wysowski DK, Fourcroy JL . Flutamide hepatotoxicity. J Urol 1996; 155: 209–212.
McLeod DG . Tolerability of nonsteroidal antiandrogens in the treatment of advanced prostate cancer. Oncologist 1997; 2: 18–27.
McCaffrey JA, Scher HI . Interstitial pneumonitis following bicalutamide treatment for prostate cancer. J Urol 1998; 160: 131.
Moinpour CM et al. Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst 1998; 90: 1537–1544.
Albertsen PC et al. Health-related quality of life among patients with metastatic prostate cancer. Urology 1997; 49: 207–216, discussion 216–217.
Naito S et al. Addition of bicalutamide 80 mg to LHRH-agonist monotherapy in patients with advanced prostate cancer: impact on quality of life. American Society of Clinical Oncology (ASCO), 5–7 June 2004, New Orleans, USA.
Moul JW et al. Epidemiology of radical prostatectomy for localized prostate cancer in the era of prostate-specific antigen: an overview of the Department of Defense Center for Prostate Disease Research national database. Surgery 2002; 132: 213–219.
Jani AB et al. Changing face and different countenances of prostate cancer: racial and geographic differences in prostate-specific antigen (PSA), stage, and grade trends in the PSA era. Int J Cancer 2001; 96: 363–371.
Messing EM et al. Immediate hormonal therapy compared with observation after radical prostatectomy and pelvic lymphadenectomy in men with node-positive prostate cancer: results at 10 years of EST 3886. Presented at AUA 2003, abstract no. 1480. Chicago, IL, USA.
Wirth MP See WA, McLeod DG. Bicalutamide ‘Casodex’ 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the Early Prostate Cancer program at 5.4 years’ median follow-up. J Urol 2004; in press.
Moul JW . Prostate specific antigen only progression of prostate cancer. J Urol 2000; 163: 1632–1642.
Pound CR et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281: 1591–1597.
Moul JW et al. Early versus delayed hormonal therapy for prostate specific antigen only recurrence of prostate cancer after radical prostatectomy. J Urol 2004; 171: 1141–1147.
European Association of Urology. EAU guidelines on prostate cancer. Available from: http://www.uroweb.org/files/uploaded_files/prostatecancer.pdf (accessed 14 May 2004).
Bolla M et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet 2002; 360: 103–106.
Pilepich MV et al. Phase III trial of androgen suppression adjuvant to definitive radiotherapy. Long term results of RTOG study 85-31. Proc Am Soc Clin Oncol 2003; 22: 381.
Roach III M et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003; 21: 1904–1911.
Author information
Authors and Affiliations
Corresponding author
Additional information
The opinions and assertions contained herein are the private views of the author and are not to be construed as the official views of the US Army or the Department of Defense.
Rights and permissions
About this article
Cite this article
Moul, J., Chodak, G. Combination hormonal therapy: a reassessment within advanced prostate cancer. Prostate Cancer Prostatic Dis 7 (Suppl 1), S2–S7 (2004). https://doi.org/10.1038/sj.pcan.4500741
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.pcan.4500741