Abstract
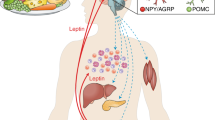
Leptin is a hormone with pleiotropic functions affecting several tissues. Because leptin has a crucial role in the adaptation of an organism to semi-starvation, anorexia nervosa (AN) serves as a model disorder to elucidate the functional implications of hypoleptinaemia; vice versa, several symptoms in patients with this eating disorder are related to the low leptin levels, which are characteristic of acute AN. Weight gain in AN patients can induce relative hyperleptinaemia in comparison to controls matched for body mass index; circulating leptin concentrations in AN patients thus transverse from subnormal to supranormal levels within a few weeks. We review findings on leptin secretion in AN and focus on implications, particularly for the hypothalamus–pituitary–gonadal axis, bone mineral density and physical hyperactivity. Undoubtedly, the elucidation of leptin's function as a trigger of diverse neuroendocrine adaptations to a restricted energy intake has substantially advanced our knowledge of the pathogenesis of distinct symptoms of AN, including amenorrhoea that represents one of the four diagnostic criteria. The fact that hypoleptinaemia can induce hyperactivity in a rat model for AN has led to a series of studies in AN patients, which support the notion that application of leptin to severely hyperactive patients might prove beneficial.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432; Erratum in: Nature 1995; 374: 479.
Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D et al. Weight-reducing effects of the plasma protein encoded by the obese gene. Science 1995; 269: 543–546.
Friedman JM . A war on obesity, not the obese. Science 2003; 299: 856–858.
Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 1999; 282: 1568–1575.
Licinio J, Mantzoros C, Negrao AB, Cizza G, Wong ML, Bongiorno PB et al. Human leptin levels are pulsatile and inversely related to pituitary–adrenal function. Nat Med 1997; 3: 575–579.
Sinha MK, Opentanova I, Ohannesian JP, Kolaczynski JW, Heiman ML, Hale J et al. Evidence of free and bound leptin in human circulation. Studies in lean and obese subjects and during short-term fasting. J Clin Invest 1996; 98: 1277–1282.
Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Eng J Med 1996; 334: 292–295.
Wabitsch M, Blum WF, Muche R, Braun M, Hube F, Rascher W et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest 1997; 100: 808–813.
Maccario M, Aimaretti G, Corneli G, Gauna C, Grottoli S, Bidlingmaier M et al. Shortterm fasting abolishes the sex-related difference in GH and leptin secretion in humans. Am J Physiol Endocrinol Metab 2000; 279: E411–E416.
Bergendahl M, Evans WS, Pastor C, Patel A, Iranmanesh A, Veldhuis JD . Short-term fasting suppresses leptin and (conversely) activates disorderly growth hormone secretion in midluteal phase women – a clinical research center study. J Clin Endocrinol Metab 1999; 84: 883–894.
Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E et al. Role of leptin in the neuroendocrine response to fasting. Nature 1996; 382: 250–252.
Farooqi S, Rau H, Whitehead J, O'Rahilly S . Ob gene mutations and human obesity. Proc Nutr Soc 1998; 57: 471–475.
Licinio J, Caglayan S, Ozata M, Yildiz BO, de Miranda PB, O'Kirwan F et al. Phenotypic effects of leptin replacement on morbid obesity, diabetes mellitus, hypogonadism, and behavior in leptin-deficient adults. Proc Natl Acad Sci USA 2004; 101: 4531–4536.
Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest 2005; 115: 3579–3586.
American Psychiatric Association. Diagnostic and Statistical Manual, 4th edn. American Psychiatric Association: Washington, DC, 1994.
Franklin JC, Schiele BC, Brozek J, Keys A . Observations on human behavior in experimental semistarvation and rehabilitation. J Clin Psychol 1948; 4: 28–45.
Casper RC, Schoeller DA, Kushner R, Hnilicka J, Gold ST . Total daily energy expenditure and activity level in anorexia nervosa. Am J Clin Nutr 1991; 53: 1143–1150.
Hebebrand J, Blum WF, Barth N, Coners H, Englaro P, Juul A et al. Leptin levels in patients with anorexia nervosa are reduced in the acute stage and elevated upon short-term weight restoration. Mol Psychiatry 1997; 2: 330–334.
Mantzoros C, Flier JS, Lesem MD, Brewerton TD, Jimerson DC . Cerebrospinal fluid leptin in anorexia nervosa: correlation with nutritional status and potential role in resistance to weight gain. J Clin Endocrinol Metab 1997; 82: 1845–1851.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-IV-TR (Text Revision), 4th edn. American Psychiatric Publishing: Washington, DC, 2000.
Hebebrand J, Himmelmann GW, Heseker H, Schafer H, Remschmidt H . Use of percentiles for the body mass index in anorexia nervosa: diagnostic, epidemiological, and therapeutic considerations. Int J Eat Disord 1996; 19: 359–369.
Hebebrand J, Wehmeier PM, Remschmidt H . Weight criteria for diagnosis of anorexia nervosa. Am J Psychiatry 2000; 157: 1024.
Hebebrand J, Casper R, Treasure J, Schweiger U . The need to revise the diagnostic criteria for anorexia nervosa. J Neural Transm 2004; 111: 827–840.
Hebebrand J, Himmelmann GW, Herzog W, Herpertz-Dahlmann BM, Steinhausen HC, Amstein M et al. Prediction of low body weight at long-term follow-up in acute anorexia nervosa by low body weight at referral. Am J Psychiatry 1997; 154: 566–569.
Casper RC, Jabine LN . An eight year follow up: outcome from adolescent compared to adult onset anorexia nervosa. J Youth Adolesc 1996; 25: 499–517.
Collins S . The limit of human adaptation to starvation. Nat Med 1995; 1: 810–814.
Hebebrand J, van der Heyden J, Devos R, Kopp W, Herpertz S, Remschmidt H et al. Plasma concentrations of obese protein in anorexia nervosa. Lancet 1995; 346: 1624–1625.
Grinspoon S, Gulick T, Askari H, Landt M, Lee K, Anderson E et al. Serum leptin levels in women with anorexia nervosa. J Clin Endocrinol Metab 1996; 81: 3861–3863.
Eckert ED, Pomeroy C, Raymond N, Kohler PF, Thuras P, Bowers CY . Leptin in anorexia nervosa. J Clin Endocrinol Metab 1998; 83: 791–795.
Wabitsch M, Ballauff A, Holl R, Blum WF, Heinze E, Remschmidt H et al. Serum leptin, gonadotropin, and testosterone concentrations in male patients with anorexia nervosa during weight gain. J Clin Endocrinol Metab 2001; 86: 2982–2988.
Mathiak K, Gowin W, Hebebrand J, Ziegler A, Blum WF, Felsenberg D et al. Serum leptin levels, body fat deposition, and weight in females with anorexia or bulimia nervosa. Horm Metab Res 1999; 31: 274–277.
Kopp W, Blum WF, von Prittwitz S, Ziegler A, Lubbert H, Emons G et al. Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol Psychiatry 1997; 2: 335–340.
Polito A, Fabbri A, Ferro-Luzzi A, Cuzzolaro M, Censi L, Ciarapica D et al. Basal metabolic rate in anorexia nervosa: relation to body composition and leptin concentrations. Am J Clin Nutr 2000; 71: 1495–1502.
Kolaczynski JW, Ohannesian JP, Considine RV, Marco CC, Caro JF . Response of leptin to short-term and prolonged overfeeding in humans. J Clin Endocrinol Metab 1996; 81: 4162–4165.
Van Harmelen V, Reynisdottir S, Eriksson P, Thorne A, Hoffstedt J, Lonnqvist F et al. Leptin secretion from subcutaneous and visceral adipose tissue in women. Diabetes 1998; 47: 913–917.
Minocci A, Savia G, Lucantoni R, Berselli ME, Tagliaferri M, Calo G et al. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord 2000; 24: 1139–1144.
Mayo-Smith W, Rosenthal DI, Goodsitt MM, Klibanski A . Intravertebral fat measurement with quantitative CT in patients with Cushing disease and anorexia nervosa. Radiology 1989; 170 (Part 1): 835–838.
Von Prittwitz S, Blum WF, Ziegler A, Scharmann S, Remschmidt H, Hebebrand J . Restrained eating is associated with low leptin levels in underweight females. Mol Psychiatry 1997; 2: 420–422.
Haas V, Onur S, Paul T, Nutzinger DO, Bosy-Westphal A, Hauer M et al. Leptin and body weight regulation in patients with anorexia nervosa before and during weight recovery. Am J Clin Nutr 2005; 81: 889–896.
Nakai Y, Hamagaki S, Kato S, Seino Y, Takagi R, Kurimoto F . Role of leptin in women with eating disorders. Int J Eat Disord 1999; 26: 29–35.
Holtkamp K, Hebebrand J, Herpertz-Dahlmann B . The contribution of anxiety and food restriction on physical activity levels in acute anorexia nervosa. Int J Eat Disord 2004; 36: 163–171.
Fichter MM, Herpertz S, Quadflieg N, Herpertz-Dahlmann B . Interview for anorexic and bulimic disorders for DSM-IV and ICD-10: updated (third) revision. Int J Eat Disord 1998; 24: 227–249.
Kolaczynski JW, Considine RV, Ohannesian J, Marco C, Opentanova I, Nyce MR et al. Responses of leptin to short-term fasting and refeeding in humans: a link with ketogenesis but not ketones themselves. Diabetes 1996; 45: 1511–1515.
Tolle V, Kadem M, Bluet-Pajot MT, Frere D, Foulon C, Bossu C et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J Clin Endocrinol Metab 2003; 88: 109–116.
Matejek N, Weimann E, Witzel C, Molenkamp G, Schwidergall S, Bohles H . Hypoleptinaemia in patients with anorexia nervosa and in elite gymnasts with anorexia athletica. Int J Sports Med 1999; 20: 451–456.
Moore SE, Morgan G, Collinson AC, Swain JA, O'Connell MA, Prentice AM . Leptin, malnutrition, and immune response in rural Gambian children. Arch Dis Child 2002; 87: 192–197.
Soliman AT, ElZalabany MM, Salama M, Ansari BM . Serum leptin concentrations during severe protein-energy malnutrition: correlation with growth parameters and endocrine function. Metabolism 2000; 49: 819–825.
Bribiescas RG . Serum leptin levels in Ache Amerindian females with normal adiposity are not significantly different from American anorexia nervosa patients. Am J Hum Biol 2005; 17: 207–210.
Holtkamp K, Hebebrand J, Mika C, Grzella I, Heer M, Heussen N et al. The effect of therapeutically induced weight gain on plasma leptin levels in patients with anorexia nervosa. J Psychiatr Res 2003; 37: 165–169.
Salisbury JJ, Levine AS, Crow SJ, Mitchell JE . Refeeding, metabolic rate, and weight gain in anorexia nervosa: a review. Int J Eat Disord 1995; 17: 337–345.
Popovic V, Djurovic M, Cetkovic A, Vojvodic D, Pekic S, Spremovic S et al. Inhibin B: a potential marker of gonadal activity in patients with anorexia nervosa during weight recovery. J Clin Endocrinol Metab 2004; 89: 1838–1843.
Djurovic M, Pekic S, Petakov M, Damjanovic S, Doknic M, Dieguez C et al. Gonadotropin response to clomiphene and plasma leptin levels in weight recovered but amenorrhoeic patients with anorexia nervosa. J Endocrinol Invest 2004; 27: 523–527.
Neuhauser-Berthold M, Herbert BM, Luhrmann PM, Sultemeier AA, Blum WF, Frey J et al. Resting metabolic rate, body composition, and serum leptin concentrations in a freeliving elderly population. Eur J Endocrinol 2000; 142: 486–492.
Mantzoros CS, Ozata M, Negrao AB, Suchard MA, Ziotopoulou M, Caglayan S et al. Synchronicity of frequently sampled thyrotropin (TSH) and leptin concentrations in healthy adults and leptin-deficient subjects: evidence for possible partial TSH regulation by leptin in humans. J Clin Endocrinol Metab 2001; 86: 3284–3291.
Kaye WH, Gwirtsman HE, Obarzanek E, George T, Jimerson DC, Ebert MH . Caloric intake necessary for weight maintenance in anorexia nervosa: nonbulimics require greater caloric intake than bulimics. Am J Clin Nutr 1986; 44: 435–443.
Holtkamp K, Hebebrand J, Mika C, Heer M, Heussen N, Herpertz-Dahlmann B . High serum leptin levels subsequent to weight gain predict renewed weight loss in patients with anorexia nervosa. Psychoneuroendocrinology 2004; 29: 791–797.
Frey J, Hebebrand J, Muller B, Ziegler A, Blum WF, Remschmidt H et al. Reduced body fat in long-term followed-up female patients with anorexia nervosa. J Psychiatr Res 2000; 34: 83–88.
Tartaglia LA . The leptin receptor. J Biol Chem 1997; 272: 6093–6096.
Baskin DG, Seeley RJ, Kuijper JL, Lok S, Weigle DS, Erickson JC et al. Increased expression of mRNA for the long form of the leptin receptor in the hypothalamus is associated with leptin hypersensitivity and fasting. Diabetes 1998; 47: 538–543.
Monteleone P, Fabrazzo M, Tortorella A, Fuschino A, Maj M . Opposite modifications in circulating leptin and soluble leptin receptor across the eating disorder spectrum. Mol Psychiatry 2002; 7: 641–646.
Misra M, Miller KK, Almazan C, Ramaswamy K, Aggarwal A, Herzog DB et al. Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. J Clin Endocrinol Metab 2004; 89: 3486–3495.
Kratzsch J, Lammert A, Bottner A, Seidel B, Mueller G, Thiery J et al. Circulating soluble leptin receptor and free leptin index during childhood, puberty, and adolescence. J Clin Endocrinol Metab 2002; 87: 4587–4594.
Jiskra J, Haluzik M, Svobodova J, Haluzikova D, Nedvidkova J, Parizkova J et al. Serum leptin levels and soluble leptin receptors in female patients with anorexia nervosa. Cas Lek Cesk 2000; 139: 660–663.
Wakeling A . Neurobiological aspects of feeding disorders. J Psychiatr Res 1985; 19: 191–201.
Chan JL, Mantzoros CS . Role of leptin in energy-deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005; 366: 74–85.
Licinio J, Negrao AB, Mantzoros C, Kaklamani V, Wong ML, Bongiorno PB et al. Synchronicity of frequently sampled, 24-h concentrations of circulating leptin, luteinizing hormone, and estradiol in healthy women. Proc Natl Acad Sci USA 1998; 95: 2541–2546.
Audi L, Mantzoros CS, Vidal-Puig A, Vargas D, Gussinye M, Carrascosa A . Leptin in relation to resumption of menses in women with anorexia nervosa. Mol Psychiatry 1998; 3: 544–547.
Ballauff A, Ziegler A, Emons G, Sturm G, Blum WF, Remschmidt H et al. Serum leptin and gonadotropin levels in patients with anorexia nervosa during weight gain. Mol Psychiatry 1999; 4: 71–75.
Di Carlo C, Tommaselli GA, De Filippo E, Pisano G, Nasti A, Bifulco G et al. Menstrual status and serum leptin levels in anorectic and in menstruating women with low body mass indexes. Fertil Steril 2002; 78: 376–382.
Chan JL, Mantzoros CS . Leptin and the hypothalamic-pituitary regulation of the gonadotropin–gonadal axis. Pituitary 2001; 4: 87–92.
Cioffi JA, Van Blerkom J, Antczak M, Shafer A, Wittmer S, Snodgrass HR . The expression of leptin and its receptors in pre-ovulatory human follicles. Mol Hum Reprod 1997; 3: 467–472.
Frisch RE, Revelle R . Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science 1970; 169: 397–399.
Holtkamp K, Mika C, Grzella I, Heer M, Pak H, Hebebrand J et al. Reproductive function during weight gain in anorexia nervosa. Leptin represents a metabolic gate to gonadotropin secretion. J Neural Transm 2003; 110: 427–435.
Treasure JL . The ultrasonographic features in anorexia nervosa and bulimia nervosa: a simplified method of monitoring hormonal states during weight gain. J Psychosom Res 1988; 32: 623–634.
Löffler S, Aust G, Köhler U, Spanel-Borowski K . Evidence of leptin expression in normal and polycystic human ovaries. Mol Hum Reprod 2001; 7: 1143–1149.
Karlsson C, Lindell K, Svensson E, Bergh C, Lind P, Billig H et al. Expression of functional leptin receptors in the human ovary. J Clin Endocrinol Metab 1997; 82: 4144–4148.
Golden NH, Jacobson MS, Schebendach J, Solanto MV, Hertz SM, Shenker IR . Resumption of menses in anorexia nervosa. Arch Pediatr Adolesc Med 1997; 151: 16–21.
Misra M, Prabhakaran R, Miller KK, Tsai P, Lin A, Lee N et al. Role of cortisol in menstrual recovery in adolescent girls with anorexia nervosa. Pediatr Res 2006; 59 (Part 1): 598–603.
Miller KK, Grinspoon S, Gleysteen S, Grieco KA, Ciampa J, Breu J et al. Preservation of neuroendocrine control of reproductive function despite severe undernutrition. J Clin Endocrinol Metab 2004; 89: 4434–4438.
Welt CK, Chan JL, Bullen J, Murphy R, Smith P, DePaoli AM et al. Recombinant human leptin in women with hypothalamic amenorrhea. N Engl J Med 2004; 351: 987–997.
Bachrach LK, Guido D, Katzman D, Litt IF, Marcus R . Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics 1990; 86: 440–447.
Rigotti NA, Neer RM, Skates SJ, Herzog DB, Nussbaum SR . The clinical course of osteoporosis in anorexia nervosa. A longitudinal study of cortical bone mass. JAMA 1991; 265: 1133–1138.
Brooks ER, Ogden BW, Cavalier DS . Compromised bone density 11.4 years after diagnosis of anorexia nervosa. J Womens Health 1998; 7: 567–574.
Katzman DK, Bachrach LK, Carter DR, Marcus R . Clinical and anthropometric correlates of bone mineral acquisition in healthy adolescent girls. J Clin Endocrinol Metab 1991; 73: 1332–1339.
Bailey DA, Martin AD, McKay HA, Whiting S, Mirwald R . Calcium accretion in girls and boys during puberty: a longitudinal analysis. J Bone Miner Res 2000; 15: 2245–2250.
Ilich JZ, Skugor M, Hangartner T, Baoshe A, Matkovic V . Relation of nutrition, body composition and physical activity to skeletal development: a cross-sectional study in preadolescent females. J Am Coll Nutr 1998; 17: 136–147.
Audi L, Vargas DM, Gussinye M, Yeste D, Marti G, Carrascosa A . Clinical and biochemical determinants of bone metabolism and bone mass in adolescent female patients with anorexia nervosa. Pediatr Res 2002; 51: 497–504.
Heer M, Mika C, Grzella I, Drummer C, Herpertz-Dahlmann B . Changes in bone turnover in patients with anorexia nervosa during eleven weeks of inpatient dietary treatment. Clin Chem 2002; 48: 754–760.
Heer M, Mika C, Grzella I, Heussen N, Herpertz-Dahlmann B . Bone turnover during inpatient nutritional therapy and outpatient follow-up in patients with anorexia nervosa compared with that in healthy control subjects. Am J Clin Nutr 2004; 80: 774–781.
Mika C, Holtkamp K, Heer M, Günther RW, Herpertz-Dahlmann B . A 2-year prospective study of bone metabolism and bone mineral density in adolescent patients with anorexia nervosa (in submission).
Elefteriou F, Takeda S, Ebihara K, Magre J, Patano N, Kim CA et al. Serum leptin level is a regulator of bone mass. Proc Natl Acad Sci USA 2004; 101: 3258–3263.
Ogueh O, Sooranna S, Nicolaides KH, Johnson MR . The relationship between leptin concentration and bone metabolism in the human fetus. J Clin Endocrinol Metab 2000; 85: 1997–1999.
Pasco JA, Henry MJ, Kotowicz MA, Collier GR, Ball MJ, Ugoni AM et al. Serum leptin levels are associated with bone mass in nonobese women. J Clin Endocrinol Metab 2001; 86: 1884–1887.
Elmquist JK, Strewler GJ . Physiology: do neural signals remodel bone? Nature 2005; 434: 447–448.
Elefteriou F . Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci 2005; 62: 2339–2349.
Pirke KM, Kellner M, Philipp E, Laessle R, Krieg JC, Fichter MM . Plasma norepinephrine after a standardized test meal in acute and remitted patients with anorexia nervosa and in healthy controls. Biol Psychiatry 1992; 31: 1074–1077.
Casper RC . Behavioral activation and lack of concern, core symptoms of anorexia nervosa? Int J Eat Disord 1998; 24: 381–393.
Exner C, Hebebrand J, Remschmidt H, Wewetzer C, Ziegler A, Herpertz S et al. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol Psychiatry 2000; 5: 476–481.
Hebebrand J, Exner C, Hebebrand K, Holtkamp C, Casper RC, Remschmidt H et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav 2003; 79: 25–37.
Holtkamp K, Herpertz-Dahlmann B, Mika C, Heer M, Heussen N, Fichter M et al. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J Clin Endocrinol Metab 2003; 88: 5169–5174.
Favaro A, Caregaro L, Burlina AB, Santonastaso P . Tryptophan levels, excessive exercise, and nutritional status in anorexia nervosa. Psychosom Med 2000; 62: 535–538.
Davis C, Katzman DK, Kaptein S, Kirsh C, Brewer H, Kalmbach et al. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr Psychiatry 1997; 38: 321–326.
Kron L, Katz JL, Gorzynski G, Weiner H . Hyperactivity in anorexia nervosa: a fundamental clinical feature. Compr Psychiatry 1978; 19: 433–440.
Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA . Leptin treatment in activitybased anorexia. Biol Psychiatry 2005a; 58: 165–171.
Kas MJ, van Dijk G, Scheurink AJ, Adan RA . Agouti-related protein prevents selfstarvation. Mol Psychiatry 2003; 8: 235–240.
Ahima RS . Central actions of adipocyte hormones. Trends Endocrinol Metab 2005; 16: 307–313.
Hillebrand JJ, Kas MJ, Scheurink AJ, van Dijk G, Adan RA . AgRP (83–132) and SHU9119 differently affect activity-based anorexia. Eur Neuropsychopharmacol 2006; 16: 403–412.
Holtkamp K, Herpertz-Dahlmann B, Hebebrand K, Mika C, Kratzsch J, Hebebrand J . Physical activity and restlessness correlate with leptin levels in patients with adolescent anorexia nervosa. Biol Psychiatry 2006; 60: 311–313.
Epling WF, Pierce WD . Activity-based anorexia in rats as a function of opportunity to run in an activity wheel. Nutr Behav 1985; 2: 37–49.
Chan JL, Matarese G, Shetty GK, Raciti P, Kelesidis I, Aufiero D et al. Differential regulation of metabolic, neuroendocrine, and immune function by leptin in humans. Proc Natl Acad Sci USA 2006; 103: 8481–8486.
Lu XY, Kim CS, Frazer A, Zhang W . Leptin: a potential novel antidepressant. Proc Natl Acad Sci USA 2006; 103: 1593–1598.
Asakawa A, Inui A, Inui T, Katsuura G, Fujino MA, Kasuga M . Leptin treatment ameliorates anxiety in ob/ob obese mice. J Diabetes Complicat 2003; 17: 105–107.
Harvey J, Shanley LJ, O'Malley D, Irving AJ . Leptin: a potential cognitive enhancer? Biochem Soc Trans 2005; 33 (Part 5): 1029–1032.
Nobili L, Baglietto MG, Beelke M, De Carli F, Di Comite R, Fiocchi I et al. Impairment of the production of delta sleep in anorectic adolescents. Sleep 2004; 27: 1553–1559.
Laposky AD, Shelton J, Bass J, Dugovic C, Perrino N, Turek FW . Altered sleep regulation in leptin-deficient mice. Am J Physiol Regul Integr Comp Physiol 2006; 290: R894–R903.
Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM et al. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J Clin Endocrinol Metab 2005; 90: 2851–2854.
Neumarker KJ, Bzufka WM, Dudeck U, Hein J, Neumarker U . Are there specific disabilities of number processing in adolescent patients with anorexia nervosa? Evidence from clinical and neuropsychological data when compared to morphometric measures from magnetic resonance imaging. Eur Child Adolesc Psychiatry 2000; 9 (Suppl 2): II111–II121.
Monteleone P, Martiadis V, Colurcio B, Maj M . Leptin secretion is related to chronicity and severity of the illness in bulimia nervosa. Psychosom Med 2002; 64: 874–879.
Misra M, Soyka LA, Miller KK, Herzog DB, Grinspoon S, De Chen D et al. Serum osteoprotegerin in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab 2003; 88: 3816–3822.
Nakai Y, Hamagaki S, Takagi R, Taniguchi A, Kurimoto F . Plasma concentrations of tumor necrosis factor-alpha (TNF-alpha) and soluble TNF receptors in patients with anorexia nervosa. J Clin Endocrinol Metab 1999; 84: 1226–1228.
Stoving RK, Vinten J, Handberg A, Ebbesen EN, Hangaard J, Hansen-Nord M et al. Diurnal variation of the serum leptin concentration in patients with anorexia nervosa. Clin Endocrinol 1998; 48: 761–768.
Acknowledgements
We are indebted to the AN patients who participated in our studies. Our studies are supported by the German National Genome Research Network.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hebebrand, J., Muller, T., Holtkamp, K. et al. The role of leptin in anorexia nervosa: clinical implications. Mol Psychiatry 12, 23–35 (2007). https://doi.org/10.1038/sj.mp.4001909
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001909
Keywords
This article is cited by
-
Unexpected identification of obesity-associated mutations in LEP and MC4R genes in patients with anorexia nervosa
Scientific Reports (2024)
-
Endocrine complications of anorexia nervosa
Journal of Eating Disorders (2023)
-
Body weight variation is not an independent factor in the determination of functional hypothalamic amenorrhea in anorexia nervosa
Journal of Endocrinological Investigation (2023)
-
“Your mind doesn’t have room for anything else”: a qualitative study of perceptions of cognitive functioning during and after recovery from anorexia nervosa
Journal of Eating Disorders (2022)
-
Genetics and neurobiology of eating disorders
Nature Neuroscience (2022)