Abstract
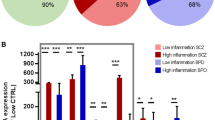
The phenomenon of adult neurogenesis (AN), that is, the generation of functional neurons from neural stem cells in the dentate gyrus of the hippocampus, has attracted remarkable attention, especially as it was shown that this process is also active in the human brain. Based on animal studies, it has been suggested that reduced AN is implicated in the etiopathology of psychiatric disorders, and that stimulation of AN contributes to the mechanism of action of antidepressant therapies. As data from human post-mortem brain are still lacking, we investigated whether the first step of AN, that is, the level of neural stem cell proliferation (NSP; as quantified by Ki-67 immunohistochemistry), is altered in tissue from the Stanley Foundation Neuropathology Consortium comprising brain specimens from patients with bipolar affective disorder, major depression, schizophrenia as well as control subjects (n=15 in each group). The hypothesis was that stem cell proliferation is reduced in affective disorders, and that antidepressant treatment increases NSP. Neither age, brain weight or pH, brain hemisphere investigated nor duration of storage had an effect on NSP. Only in bipolar disorder, post-mortem interval was a significant intervening variable. In disease, onset of the disorder and its duration likewise did not affect NSP. Also, cumulative lifetime dose of fluphenazine was not correlated with NSP, and presence of antidepressant treatment did not result in an increase of NSP. Concerning the different diagnostic entities, reduced amounts of newly formed cells were found in schizophrenia, but not in major depression. Our findings suggest that reduced NSP may contribute to the pathogenesis of schizophrenia, whereas the rate of NSP does not seem to be critical to the etiopathology of affective disorders, nor is it modified by antidepressant drug treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Song HJ, Stevens CF, Gage FH . Neural stem cells from adult hippocampus develop essential properties of functional CNS neurons. Nat Neurosci 2002; 5: 438–445.
van Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH . Functional neurogenesis in the adult hippocampus. Nature 2002; 415: 1030–1034.
Kempermann G . Why new neurons? Possible functions for adult hippocampal neurogenesis. J Neurosci 2002; 22: 635–638.
Duman RS, Malberg J, Nakagawa S . Regulation of adult neurogenesis by psychotropic drugs and stress. J Pharmacol Exp Ther 2001; 299: 401–407.
Duman RS . Depression: a case of neuronal life and death? Biol Psychiatry 2004; 56: 140–145.
Benninghoff J, Schmitt A, Mossner R, Lesch KP . When cells become depressed: focus on neural stem cells in novel treatment strategies against depression. J Neural Transm 2002; 109: 947–962.
Kempermann G, Kronenberg G . Depressed new neurons – adult hippocampal neurogenesis and a cellular plasticity hypothesis of major depression. Biol Psychiatry 2003; 54: 499–503.
Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrie P . Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 2004; 9: 278–286, 224.
Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry 2003; 54: 1025–1034.
Gould E, Tanapat P . Stress and hippocampal neurogenesis. Biol Psychiatry 1999; 46: 1472–1479.
Mirescu C, Peters JD, Gould E . Early life experience alters response of adult neurogenesis to stress. Nat Neurosci 2004; 7: 841–846.
van Praag H, Kempermann G, Gage FH . Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999; 2: 266–270.
Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingstrom A . Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry 2000; 47: 1043–1049.
Vollmayr B, Simonis C, Weber S, Gass P, Henn F . Reduced cell proliferation in the dentate gyrus is not correlated with the development of learned helplessness. Biol Psychiatry 2003; 54: 1035–1040.
Pham K, Nacher J, Hof PR, McEwen BS . Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 2003; 17: 879–886.
Reif A, Schmitt A, Fritzen S, Chourbaji S, Bartsch C, Urani A et al. Differential effect of endothelial nitric oxide synthase (NOS-III) on the regulation of adult neurogenesis and behaviour. Eur J Neurosci 2004; 20: 885–895.
Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805–809.
Geuze E, Vermetten E, Bremner JD . MR-based in vivo hippocampal volumetrics: 2. Findings in neuropsychiatric disorders. Mol Psychiatry 2005; 10: 160–184.
Videbech P, Ravnkilde B . Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004; 161: 1957–1966.
Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY et al. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiatry 2004; 56: 640–650.
Lucassen PJ, Muller MB, Holsboer F, Bauer J, Holtrop A, Wouda J et al. Hippocampal apoptosis in major depression is a minor event and absent from subareas at risk for glucocorticoid overexposure. Am J Pathol 2001; 158: 453–468.
Muller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF . Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci 2001; 14: 1603–1612.
Benes FM, Kwok EW, Vincent SL, Todtenkopf MS . A reduction of nonpyramidal cells in sector CA2 of schizophrenics and manic depressives. Biol Psychiatry 1998; 44: 88–97.
Curtis MA, Penney EB, Pearson AG, van Roon-Mom WM, Butterworth NJ, Dragunow M et al. Increased cell proliferation and neurogenesis in the adult human Huntington's disease brain. Proc Natl Acad Sci USA 2003; 100: 9023–9027.
Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 2004; 7: 726–735.
Jin K, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC et al. Increased hippocampal neurogenesis in Alzheimer's disease. Proc Natl Acad Sci USA 2004; 101: 343–347.
Kee N, Sivalingam S, Boonstra R, Wojtowicz JM . The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 2002; 115: 97–105.
Torrey EF, Webster M, Knable M, Johnston N, Yolken RH . The stanley foundation brain collection and neuropathology consortium. Schizophr Res 2000; 44: 151–155.
Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA et al. Neurogenesis in the adult human hippocampus. Nat Med 1998; 4: 1313–1317.
Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, Collado-Morente L, Muriach M et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proc Natl Acad Sci USA 2003; 100: 7919–7924.
Scott BW, Wojtowicz JM, Burnham WM . Neurogenesis in the dentate gyrus of the rat following electroconvulsive shock seizures. Exp Neurol 2000; 165: 231–236.
Ohta Y, Ichimura K . Proliferation markers, proliferating cell nuclear antigen, Ki67, 5-bromo-2′-deoxyuridine, and cyclin D1 in mouse olfactory epithelium. Ann Otol Rhinol Laryngol 2000; 109: 1046–1048.
Muskhelishvili L, Latendresse JR, Kodell RL, Henderson EB . Evaluation of cell proliferation in rat tissues with BrdU, PCNA, Ki-67(MIB-5) immunohistochemistry and in situ hybridization for histone mRNA. J Histochem Cytochem 2003; 51: 1681–1688.
Scholzen T, Gerdes J . The Ki-67 protein: from the known and the unknown. J Cell Physiol 2000; 182: 311–322.
Kuan CY, Schloemer AJ, Lu A, Burns KA, Weng WL, Williams MT et al. Hypoxia–ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci 2004; 24: 10763–10772.
Hellsten J, Wennstrom M, Bengzon J, Mohapel P, Tingstrom A . Electroconvulsive seizures induce endothelial cell proliferation in adult rat hippocampus. Biol Psychiatry 2004; 55: 420–427.
Hellsten J, Wennstrom M, Mohapel P, Ekdahl CT, Bengzon J, Tingstrom A . Electroconvulsive seizures increase hippocampal neurogenesis after chronic corticosterone treatment. Eur J Neurosci 2002; 16: 283–290.
Kodama M, Fujioka T, Duman RS . Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry 2004; 56: 570–580.
Henn FA, Vollmayr B . Neurogenesis and depression: etiology or epiphenomenon? Biol Psychiatry 2004; 56: 146–150.
Keilhoff G, Bernstein HG, Becker A, Grecksch G, Wolf G . Increased neurogenesis in a rat ketamine model of schizophrenia. Biol Psychiatry 2004; 56: 317–322.
Wakade CG, Mahadik SP, Waller JL, Chiu FC . Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res 2002; 69: 72–79.
Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ et al. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry 2001; 58: 829–835.
Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK . Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology 2004; 29: 1063–1069.
Wang HD, Dunnavant FD, Jarman T, Deutch AY . Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology 2004; 29: 1230–1238.
Kurtz MM . Neurocognitive impairment across the lifespan in schizophrenia: an update. Schizophr Res 2005; 74: 15–26.
Prickaerts J, Koopmans G, Blokland A, Scheepens A . Learning and adult neurogenesis: survival with or without proliferation? Neurobiol Learn Mem 2004; 81: 1–11.
Kempermann G, Kuhn HG, Gage FH . More hippocampal neurons in adult mice living in an enriched environment. Nature 1997; 386: 493–495.
Berrettini W . Evidence for shared susceptibility in bipolar disorder and schizophrenia. Am J Med Genet C 2003; 123: 59–64.
Beckmann H, Jakob H . Prenatal disturbances of nerve cell migration in the entorhinal region: a common vulnerability factor in functional psychoses? J Neural Transm Gen Sect 1991; 84: 155–164.
Acknowledgements
We thank T Töpner for excellent technical assistance. Post-mortem brain tissue was donated by The Stanley Medical Research Institute Brain Collection, courtesy of Drs Michael B Knable, E Fuller Torrey, Maree J Webster and Robert H Yolken. This study was supported by the Deutsche Forschungsgemeinschaft (Grant RE16321-1 to AR and ML, KFO 1251-1 D to AR and KPL and SFB 581 to KPL), BMBF (IZKF 01 KS 9603) and the European Commission (NEWMOOD LSHM-CT-2003-503474).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reif, A., Fritzen, S., Finger, M. et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11, 514–522 (2006). https://doi.org/10.1038/sj.mp.4001791
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001791
Keywords
This article is cited by
-
Molecular mapping of a core transcriptional signature of microglia-specific genes in schizophrenia
Translational Psychiatry (2023)
-
Major depressive disorder
Nature Reviews Disease Primers (2023)
-
Schizophrenia in the genetic era: a review from development history, clinical features and genomic research approaches to insights of susceptibility genes
Metabolic Brain Disease (2023)
-
Growth rates of human induced pluripotent stem cells and neural stem cells from attention-deficit hyperactivity disorder patients: a preliminary study
Journal of Neural Transmission (2023)
-
Hippocampal circuit dysfunction in psychosis
Translational Psychiatry (2022)