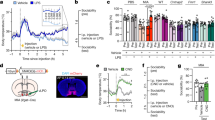
Abstract
The developing brain is uniquely susceptible to the neurotoxic hazard posed by mercurials. Host differences in maturation, metabolism, nutrition, sex, and autoimmunity influence outcomes. How population-based variability affects the safety of the ethylmercury-containing vaccine preservative, thimerosal, is unknown. Reported increases in the prevalence of autism, a highly heritable neuropsychiatric condition, are intensifying public focus on environmental exposures such as thimerosal. Immune profiles and family history in autism are frequently consistent with autoimmunity. We hypothesized that autoimmune propensity influences outcomes in mice following thimerosal challenges that mimic routine childhood immunizations. Autoimmune disease-sensitive SJL/J mice showed growth delay; reduced locomotion; exaggerated response to novelty; and densely packed, hyperchromic hippocampal neurons with altered glutamate receptors and transporters. Strains resistant to autoimmunity, C57BL/6J and BALB/cJ, were not susceptible. These findings implicate genetic influences and provide a model for investigating thimerosal-related neurotoxicity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Risch N, Spiker D, Lotspeich L, Nouri N, Hinds D, Hallmayer J et al. A genomic screen of autism: evidence for a multilocus etiology. Am J Hum Genet 1999; 65: 493–507.
Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C . Prevalence of autism in a US metropolitan area. JAMA 2003; 289: 49–55.
M.I.N.D Institute. Report to the Legislature on the Principal Findings from the Epidemiology of Autism in California: A Comprehensive Pilot Study. UC Davis: Sacramento, CA, 2002.
Chakrabarti S, Fombonne E . Pervasive developmental disorders in preschool children. JAMA 2001; 285: 3093–3099.
Blaxill MF, Baskin DS, Spitzer WO . Commentary: Blaxill, Baskin, and Spitzer on Croen et al. (2002). The changing prevalence of autism in California. J Autism Dev Disord 2003; 33: 223–226.
Croen L, Grether J . Response: a response to Blaxill, Baskin, and Spitzer on Croen et al. (2002). The changing prevalence of autism in California. J Autism Dev Disord 2003; 33: 227–229.
American Academy of Pediatrics Committee on Infectious Diseases. Recommended childhood immunization schedule—United States, January–December 2001. Pediatrics 2001; 107: 202–204.
Committee on the Toxicological Effects of Methylmercury, Board on Environmental Studies and Toxicology, National Research Council. Toxicological Effects of Methylmercury. National Academies Press: Washington, DC, 2000.
Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB et al. Safety of thimerosal-containing vaccines: a two-phased study of computerized health maintenance organization databases. Pediatrics 2003; 112: 1039–1048.
Connolly AM, Chez MG, Pestronk A, Arnold ST, Mehta S, Deuel RK . Serum autoantibodies to brain in Landau–Kleffner variant, autism, and other neurologic disorders. J Pediatrics 1999; 134: 607–613.
Vojdani A, Pangborn JB, Vojdani E, Cooper EL . Infections, toxic chemicals and dietary peptides binding to lymphocyte receptors and tissue enzymes are major instigators of autoimmunity in autism. Int J Immunopathol Pharmacol 2003; 16: 189–199.
Singh VK, Warren RP, Odell JD, Warren WL, Cole P . Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun 1993; 7: 93–103.
Warren RP, Odell JD, Warren WL, Burger RA, Maciulis A, Daniels WW et al. Strong association of the third hypervariable region of HLA-DR beta 1 with autism. J Neuroimmunol 1996; 67: 97–102.
Torres AR, Maciulis A, Stubbs EG, Cutler A, Odell D . The transmission disequilibrium test suggests that HLA-DR4 and DR13 are linked to autism spectrum disorder. Hum Immunol 2002; 63: 311–316.
Warren RP, Yonk J, Burger RW, Odell D, Warren WL . DR-positive T cells in autism: association with decreased plasma levels of the complement C4B protein. Neuropsychobiology 1995; 31: 53–57.
Sweeten TL, Bowyer SL, Posey DJ, Halberstadt GM, McDougle CJ . Increased prevalence of familial autoimmunity in probands with pervasive developmental disorders. Pediatrics 2003; 112: e420.
Comi AM, Zimmerman AW, Frye VH, Law PA, Peeden JN . Familial clustering of autoimmune disorders and evaluation of medical risk factors in autism. J Child Neurol 1999; 14: 388–394.
Hultman P, Hansson-Georgiadis H . Methyl mercury-induced autoimmunity in mice. Toxicol Appl Pharmacol 1999; 154: 203–211.
Abedi-Valugerdi M, Möller G . Contribution of H-2 and non-H-2 genes in the control of mercury-induced autoimmunity. Int Immunol 2000; 12: 1425–1430.
Klein J, Benoist C, David CS, Demant P, Lindahl KF, Flaherty L et al. Revised nomenclature of mouse H-2 genes. Immunogenetics 1990; 32: 147–149.
Rice D, Barone SJ . Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000; 108: 511–533.
Holladay SD, Smialowicz RJ . Development of the murine and human immune system: differential effects of immunotoxicants depend on time of exposure. Environ Health Perspect 2000; 108: 463–473.
Fink GR, Zilles K, Schleicher A . Postnatal development of forebrain regions in the autoimmune NZB-mouse. A model for degeneration in neuronal systems. Anat Embryol (Berl) 1991; 183: 579–588.
National Center for Health Statistics. Birth to 36 Months: Boys Length-for-Age and Weight-for-Age Percentiles. CDC: Atlanta, GA, 2000.
Altman J, Sudarshan K . Postnatal development of locomotion in the laboratory rat. Anim Behav 1975; 23: 896–920.
Holson RR, Pearce B . Principle and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol 1992; 14: 221–228.
Cory-Slechta DA, Crofton KM, Foran JA, Ross JF, Sheets LP, Weiss B et al. Methods to identify and characterize developmental neurotoxicity for human health risk assessment. I: Behavioral effects. Environ Health Perspect 2001; 109(Suppl 1): 79–91.
Paxinos G, Franklin KBJ . The Mouse Brain in Stereotaxic Coordinates. Academic Press: San Diego, CA, 2001.
Gavrieli Y, Sherman Y, Ben-Sasson SA . Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992; 119: 493–501.
Pichichero ME, Cernichiari E, Lopreiato J, Treanor J . Mercury concentrations and metabolism in infants receiving vaccines containing thiomersal: a descriptive study. Lancet 2002; 360: 1737–1741.
Sager PR . Evaluation of thimerosal-containing vaccines in non-human primates. Presentation at CDC, ACIP Meeting, 19 June 2003.
Magos L, Brown AW, Sparrow S, Bailey E, Snowden RT, Skipp WR . The comparative toxicology of ethyl- and methylmercury. Arch Toxicol 1985; 57: 260–267.
Oskarsson A, Palminger Hallen I, Sundberg J, Petersson Grawe K . Risk assessment in relation to neonatal metal exposure. Analyst 1998; 123: 19–23.
Havarinasab S, Lambertsson L, Qvarnstrom J, Hultman P . Dose–response study of thimerosal-induced murine systemic autoimmunity. Toxicol Appl Pharmacol 2004; 194: 169–179.
Hultman P, Bell LJ, Enestrom S, Pollard KM . Murine susceptibility to mercury. I. Autoantibody profiles and systemic immune deposits in inbred, congenic, and intra-H-2 recombinant strains. Clin Immunol Immunopathol 1992; 65: 98–109.
Hultman P, Bell LJ, Enestrom S, Pollard KM . Murine susceptibility to mercury. II. Autoantibody profiles and renal immune deposits in hybrid, backcross, and H-2d congenic mice. Clin Immunol Immunopathol 1993; 68: 9–20.
Hanley GA, Schiffenbauer J, Sobel ES . Resistance to HgCl2-induced autoimmunity in haplotype–heterozygous mice is an intrinsic property of B cells. J Immunol 1998; 161: 1778–1785.
Johansson U, Hansson-Georgiadis H, Hultman P . The genotype determines the B cell response in mercury-treated mice. Int Arch Allergy Immunol 1998; 116: 295–305.
Pollard KM, Pearson DL, Hultman P, Deane TN, Lindh U, Kono DH . Xenobiotic acceleration of idiopathic systemic autoimmunity in lupus-prone BXSB mice. Environ Health Perspect 2001; 109: 27–33.
Nielsen JB, Hultman P . Mercury-induced autoimmunity in mice. Environ Health Perspect 2002; 110(Suppl 5): 877–881.
Nielsen JB . Toxicokinetics of mercuric chloride and methylmercuric chloride in mice. J Toxicol Environ Health 1992; 37: 85–122.
Hultman P, Nielsen JB . The effect of toxicokinetics on murine mercury-induced autoimmunity. Environ Res 1998; 77: 141–148.
Nielsen JB, Andersen O . Methyl mercuric chloride toxicokinetics in mice. I: Effects of strain, sex, route of administration and dose. Pharmacol Toxicol 1991; 68: 201–207.
Hirayama K, Yasutake A . Sex and age differences in mercury distribution and excretion in methylmercury-administered mice. J Toxicol Environ Health 1986; 18: 49–60.
Westphal GA, Schnuch A, Schulz TG, Reich K, Aberer W, Brasch J et al. Homozygous gene deletions of the glutathione S-transferases M1 and T1 are associated with thimerosal sensitization. Int Arch Occup Environ Health 2000; 73: 384–388.
Waly M, Olteanu H, Banerjee R, Choi SW, Mason JB, Parker BS et al. Activation of methionine synthase by insulin-like growth factor-1 and dopamine: a target for neurodevelopmental toxins and thimerosal. Mol Psychiatry 2004; 9: 358–370.
Maas K, Chan S, Parker J, Slater A, Moore J, Olsen N et al. Cutting edge: molecular portrait of human autoimmune disease. J Immunol 2002; 169: 5–9.
Schauwecker PE . Modulation of cell death by mouse genotype: differential vulnerability to excitatory amino acid-induced lesions. Exp Neurol 2002; 178: 219–235.
Espejo C, Carrasco J, Hidalgo J, Penkowa M, Garcia A, Saez-Torres I et al. Differential expression of metallothioneins in the CNS of mice with experimental autoimmune encephalomyelitis. Neuroscience 2001; 105: 1055–1065.
Wilson AG, di Giovine FS, Duff GW . Genetics of tumour necrosis factor-alpha in autoimmune, infectious, and neoplastic diseases. J Inflamm 1995; 45: 1–12.
Kono DH, Balomenos D, Pearson DL, Park MS, Hildebrandt B, Hultman P et al. The prototypic Th2 autoimmunity induced by mercury is dependent on IFN-gamma and not Th1/Th2 imbalance. J Immunol 1998; 161: 234–240.
Hill N, Sarvetnick N . Cytokines: promoters and dampeners of autoimmunity. Curr Opin Immunol 2002; 14: 791–797.
Charles PC, Weber KS, Cipriani B, Brosnan CF . Cytokine, chemokine and chemokine receptor mRNA expression in different strains of normal mice: implications for establishment of a Th1/Th2 bias. J Neuroimmunol 1999; 100: 64–73.
Billiau A, Heremans H, Vandekerckhove F, Dijkmans R, Sobis H, Meulepas E et al. Enhancement of experimental allergic encephalomyelitis in mice by antibodies against IFN-gamma. J Immunol 1988; 140: 1506–1510.
Butterfield RJ, Sudweeks JD, Blankenhorn EP, Korngold R, Marini JC, Todd JA, Roper RJ, Teuscher C . New genetic loci that control susceptibility and symptoms of experimental allergic encephalomyelitis in inbred mice. J Immunol 1998; 161: 1860–1867.
Krakowski M, Owens T . Interferon-gamma confers resistance to experimental allergic encephalomyelitis. Eur J Immunol 1996; 26: 1641–1646.
Hafidi A, Hillman DE . Distribution of glutamate receptors GluR 2/3 and NR1 in the developing rat cerebellum. Neuroscience 1997; 81: 427–436.
Olney JW . New insights and new issues in developmental neurotoxicology. Neurotoxicology 2002; 23: 659–668.
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K et al. Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 1999; 283: 70–74.
Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY . Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999; 23: 185–188.
Bauman ML, Kemper TL, Arin DM . Microscopic observations of the brain in Rett syndrome. Neuropediatrics 1995; 26: 105–108.
Leontovich TA, Mukhina JK, Fedorov AA, Belichenko PV . Morphological study of the entorhinal cortex, hippocampal formation, and basal ganglia in Rett syndrome patients. Neurobiol Dis 1999; 6: 77–91.
Courchesne E, Karns CM, Davis HR, Ziccardi R, Carper RA, Tigue ZD et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology 2001; 57: 245–254.
Deutsch CK, Saunders E, Lauer EA, Joseph R, Tager-Flusberg H . Quantitative assessment of craniofacial dysmorphology in autism and SLI. Presentation at International Meeting for Autism Research. Orlando, FL, Vol. 2, 2002; 44.
Kantor DB, Kolodkin AL . Curbing the excesses of youth: molecular insights into axonal pruning. Neuron 2003; 38: 849–852.
Crusio WE . Genetic dissection of mouse exploratory behaviour. Behav Brain Res 2001; 125: 127–132.
Furuta A, Noda M, Suzuki SO, Goto Y, Kanahori Y, Rothstein JD et al. Translocation of glutamate transporter subtype excitatory amino acid carrier 1 protein in kainic acid-induced rat epilepsy. Am J Pathol 2003; 163: 779–787.
Proper EA, Hoogland G, Kappen SM, Jansen GH, Rensen MG, Schrama LH et al. Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 2002; 125: 32–43.
Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D et al. Localization of neuronal and glial glutamate transporters. Neuron 1994; 13: 713–725.
Conti F, DeBiasi S, Minelli A, Rothstein JD, Melone M . EAAC1, a high-affinity glutamate transporter, is localized to astrocytes and GABAergic neurons besides pyramidal cells in the rat cerebral cortex. Cereb Cortex 1998; 8: 108–116.
Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R et al. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci 2002; 22: 6372–6379.
Furuta A, Rothstein JD, Martin LJ . Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci 1997; 17: 8363–8375.
Geddes JW, Brunner L, Cotman CW, Buzsaki G . Alterations in [3H]kainate and N-methyl-D-aspartate-sensitive L-[3H]-glutamate binding in the rat hippocampal formation following fimbria–fornix lesions. Exp Neurol 1992; 115: 271–281.
Franck JE, Kunkel DD, Baskin DG, Schwartzkroin PA . Inhibition in kainate-lesioned hyperexcitable hippocampi: physiologic, autoradiographic, and immunocytochemical observations. J Neurosci 1988; 8: 1991–2002.
Brady RJ, Gorter JA, Monroe MT, Swann JW . Developmental alterations in the sensitivity of hippocampal NMDA receptors to AP5. Brain Res Dev Brain Res 1994; 83: 190–196.
Acknowledgements
This work was supported by grants from UC Davis M.I.N.D. Institute (MH), Coalition for Safe Minds (MH), The Ellison Medical Foundation (WIL), and NIH HD37546 (WIL). The technical assistance of Janelle Villiers, Arya Soman, and Peter Hardigan is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hornig, M., Chian, D. & Lipkin, W. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry 9, 833–845 (2004). https://doi.org/10.1038/sj.mp.4001529
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4001529
Keywords
This article is cited by
-
Propofol toxicity in the developing mouse heart mitochondria
Pediatric Research (2022)
-
Effects of Treadmill Exercise on the Expression Level of BAX, BAD, BCL-2, BCL-XL, TFAM, and PGC-1α in the Hippocampus of Thimerosal-Treated Rats
Neurotoxicity Research (2021)
-
The Novel Potential Therapeutic Utility of Montelukast in Alleviating Autistic Behavior Induced by Early Postnatal Administration of Thimerosal in Mice
Cellular and Molecular Neurobiology (2021)
-
Microbial structure and function in infant and juvenile rhesus macaques are primarily affected by age, not vaccination status
Scientific Reports (2018)
-
Levels of Metals in the Blood and Specific Porphyrins in the Urine in Children with Autism Spectrum Disorders
Biological Trace Element Research (2015)