Abstract
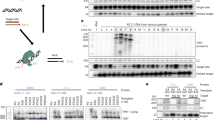
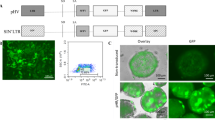
Gene transfer vectors encoding two or more genes are potentially powerful research tools and are poised to play an increasingly important role in gene therapy applications. Common strategies employed to express more than one transgene per vector include the use of multiple promoters, internal ribosome entry site (IRES) elements, splicing signals and fusion proteins. Of these, the IRES elements and multiple promoters have been most widely used. The use of multiple promoters, however, may be compromised by interference between promoters, promoter silencing and vector rearrangements or deletions. In this study, we demonstrate promoter interference between two internal heterologous promoters in the context of a late-generation lentiviral vector. The interference, involving the human cytomegalovirus-immediate-early promoter and human elongation-factor-1α promoter, occurred bidirectionally with both promoters markedly impairing expression of the adjacent transcription unit. The data presented not only highlight the potential for interference between these widely-used promoters, but also the value of a sequential approach to vector construction that allows such effects to be recognized.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Gao H, Xiao J, Sun Q, Lin H, Bai Y, Yang L et al. A single decoy oligodeoxynucleotides targeting multiple oncoproteins produces strong anticancer effects. Mol Pharmacol 2006; 70: 1621–1629.
Zhong S, Liu C, Haviland D, Doris PA, Teng BB . Simultaneous expression of apolipoprotein B mRNA editing enzyme and scavenger receptor BI mediated by a therapeutic gene expression system. Atherosclerosis 2006; 184: 264–275.
Wang HJ, Yu CL, Kishi H, Motoki K, Mao ZB, Muraguchi A . Suppression of experimental osteoarthritis by adenovirus-mediated double gene transfer. Chin Med J (Engl) 2006; 119: 1365–1373.
Ghattas IR, Sanes JR, Majors JE . The encephalomyocarditis virus internal ribosome entry site allows efficient coexpression of two genes from a recombinant provirus in cultured cells and in embryos. Mol Cell Biol 1991; 11: 5848–5859.
De Felipe P . Polycistronic viral vectors. Curr Gene Ther 2002; 2: 355–378.
Ngoi SM, Chien AC, Lee CG . Exploiting internal ribosome entry sites in gene therapy vector design. Curr Gene Ther 2004; 4: 15–31.
De Felipe P, Izquierdo M . Construction and characterization of pentacistronic retrovirus vectors. J Gen Virol 2003; 84: 1281–1285.
Allera-Moreau C, Chomarat P, Audinot V, Coge F, Gillard M, Martineau Y et al. The use of IRES-based bicistronic vectors allows the stable expression of recombinant G-protein coupled receptors such as NPY5 and histamine 4. Biochimie 2006; 88: 737–746.
Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T . IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther 2000; 1: 376–382.
Berberich SL, Stoltzfus CM . Mutations in the regions of the Rous sarcoma virus 3′ splice sites: implications for regulation of alternative splicing. J Virol 1991; 65: 2640–2646.
Germann UA, Chin KV, Pastan I, Gottesman MM . Retroviral transfer of a chimeric multidrug resistance-adenosine deaminase gene. FASEB J 1990; 4: 1501–1507.
Ryan MD, Drew J . Foot-and-mouth disease virus 2A oligopeptide mediated cleavage of an artificial polyprotein. EMBO J 1994; 13: 928–933.
Furler S, Paterna JC, Weibel M, Bueler H . Recombinant AAV vectors containing the foot and mouth disease virus 2A sequence confer efficient bicistronic gene expression in cultured cells and rat substantia nigra neurons. Gene Ther 2001; 8: 864–873.
Eszterhas SK, Bouhassira EE, Martin DI, Fiering S . Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol Cell Biol 2002; 22: 469–479.
Cullen BR, Lomedico PT, Ju G . Transcriptional interference in avian retroviruses—implications for the promoter insertion model of leukaemogenesis. Nature 1984; 307: 241–245.
Emerman M, Temin HM . Genes with promoters in retrovirus vectors can be independently suppressed by an epigenetic mechanism. Cell 1984; 39: 449–467.
Emerman M, Temin HM . High-frequency deletion in recovered retrovirus vectors containing exogenous DNA with promoters. J Virol 1984; 50: 42–49.
Hildinger M, Schilz A, Eckert HG, Bohn W, Fehse B, Zander A et al. Bicistronic retroviral vectors for combining myeloprotection with cell-surface marking. Gene Ther 1999; 6: 1222–1230.
Edelstein ML, Abedi MR, Wixon J, Edelstein RM . Gene therapy clinical trials worldwide 1989–2004-an overview. J Gene Med 2004; 6: 597–602.
Reiser J, Lai Z, Zhang XY, Brady RO . Development of multigene and regulated lentivirus vectors. J Virol 2000; 74: 10589–10599.
Naldini L, Blomer U, Gallay P, Ory D, Mulligan R, Gage FH et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996; 272: 263–267.
Naldini L, Blomer U, Gage FH, Trono D, Verma IM . Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci USA 1996; 93: 11382–11388.
Adam MA, Ramesh N, Miller AD, Osborne WR . Internal initiation of translation in retroviral vectors carrying picornavirus 5′ nontranslated regions. J Virol 1991; 65: 4985–4990.
Monahan PE, Samulski RJ . AAV vectors: is clinical success on the horizon? Gene Ther 2000; 7: 24–30.
Wu X, Li Y, Crise B, Burgess SM . Transcription start regions in the human genome are favored targets for MLV integration. Science 2003; 300: 1749–1751.
Mitchell RS, Beitzel BF, Schroder AR, Shinn P, Chen H, Berry CC et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol 2004; 2: E234.
Hematti P, Hong BK, Ferguson C, Adler R, Hanawa H, Sellers S et al. Distinct genomic integration of MLV and SIV vectors in primate hematopoietic stem and progenitor cells. PLoS Biol 2004; 2: e423.
Yu X, Zhan X, D’Costa J, Tanavde VM, Ye Z, Peng T et al. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther 2003; 7: 827–838.
Ben Dor I, Itsykson P, Goldenberg D, Galun E, Reubinoff BE . Lentiviral vectors harboring a dual-gene system allow high and homogeneous transgene expression in selected polyclonal human embryonic stem cells. Mol Ther 2006; 14: 255–267.
Ginn SL, Fleming J, Rowe PB, Alexander IE . Promoter interference mediated by the U3 region in early-generation HIV-1-derived lentivirus vectors can influence detection of transgene expression in a cell-type and species-specific manner. Hum Gene Ther 2003; 14: 1127–1137.
Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol 1998; 72: 9873–9880.
Greger IH, Demarchi F, Giacca M, Proudfoot NJ . Transcriptional interference perturbs the binding of Sp1 to the HIV-1 promoter. Nucleic Acids Res 1998; 26: 1294–1301.
Isomura H, Stinski MF, Kudoh A, Daikoku T, Shirata N, Tsurumi T . Two Sp1/Sp3 binding sites in the major immediate-early proximal enhancer of human cytomegalovirus have a significant role in viral replication. J Virol 2005; 79: 9597–9607.
Uetsuki T, Naito A, Nagata S, Kaziro Y . Isolation and characterization of the human chromosomal gene for polypeptide chain elongation factor-1 alpha. J Biol Chem 1989; 264: 5791–5798.
Tolmachov O, Ma YL, Themis M, Patel P, Spohr H, MacLeod KT et al. Overexpression of connexin 43 using a retroviral vector improves electrical coupling of skeletal myoblasts with cardiac myocytes in vitro. BMC Cardiovasc Disord 2006; 6: 25.
Gaszner M, Felsenfeld G . Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet 2006; 7: 703–713.
Kuhn EJ, Geyer PK . Genomic insulators: connecting properties to mechanism. Curr Opin Cell Biol 2003; 15: 259–265.
Follenzi A, Ailles LE, Bakovic S, Geuna M, Naldini L . Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat Genet 2000; 25: 217–222.
Graham FL, Smiley J, Russell WC, Nairn R . Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol 1977; 36: 59–74.
Sastry L, Johnson T, Hobson MJ, Smucker B, Cornetta K . Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther 2002; 9: 1155–1162.
Smyth CM, Ginn SL, Deakin CT, Logan GJ, Alexander IE . Limiting γc expression differentially affects signaling via the interleukin (IL)-7 and IL-15 receptors. Blood 2007; 110: 91–98.
Acknowledgements
We thank Professor Inder Verma (Salk Institute, San Diego, USA) for providing the lentiviral vector reagents used in this study. This work was supported by an ASTRA fellowship from the Royal Australasian Collage of Physicians and by the Haemophilia Foundation of Australia. Julie A Curtin was the recipient of a NHMRC post-graduate research scholarship and a CHW New Investigator Grant. We also thank ACCO Australia, for funding support. Samantha L Ginn is the recipient of a fellowship honouring the memory of Noel Dowling.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Curtin, J., Dane, A., Swanson, A. et al. Bidirectional promoter interference between two widely used internal heterologous promoters in a late-generation lentiviral construct. Gene Ther 15, 384–390 (2008). https://doi.org/10.1038/sj.gt.3303105
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303105
Keywords
This article is cited by
-
A lentiviral vector for the production of T cells with an inducible transgene and a constitutively expressed tumour-targeting receptor
Nature Biomedical Engineering (2023)
-
CAR-T cell potency: from structural elements to vector backbone components
Biomarker Research (2022)
-
Combinatorial gene targeting in primary human hematopoietic stem and progenitor cells
Scientific Reports (2022)
-
Reciprocal transactivation of Merkel cell polyomavirus and high-risk human papillomavirus promoter activities and increased expression of their oncoproteins
Virology Journal (2021)
-
‘Mini’ U6 Pol III promoter exhibits nucleosome redundancy and supports multiplexed coupling of CRISPR/Cas9 effects
Gene Therapy (2020)